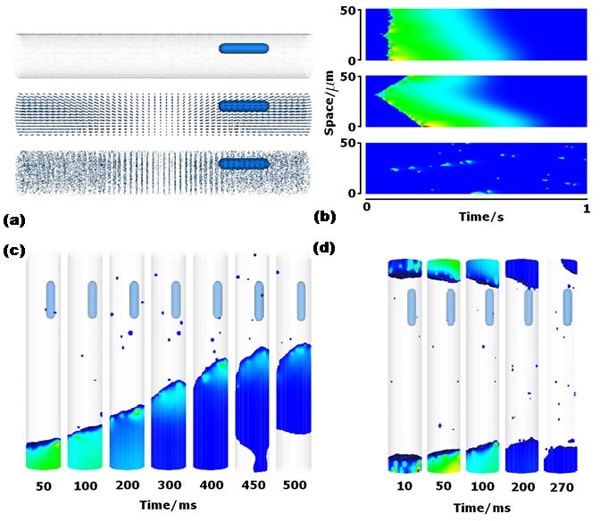
To investigate the mechanisms underlying the initiation and propagation of intracellular calcium wave, we developed a three-dimensional ventricular e-cell (3DVe-cell), where cell membrane, nucleus, ryanodine receptor clusters, Z-disk, cytoplasm are modeled as spatially distributed structures. The geometry of the 3DVe-cell is taken as a cylinder with diameter of 16 μm and length of 100 μm with the space step of 0.2 μm, which gives 80 by 80 by 500 3D matrix with 3.2 million grid points. RyR clusters are spatially distributed based on [1]. The detailed mathematical description of calcium sparks and calcium waves is a set of reaction-diffusion equations based on [2]. A normal excitation of the cardiac cell produces the membrane depolarization of the action potential. Ca2+ influxes (Ca2+ current through channels) predominantly in the T tubules activate most of the CRUs and incur SR releases, and then free cytosolic calcium concentration arises uniformly throughout the cell. In Figure (a), pseudo multi linescans of transverse section of the cell are obtained under different conditions, such as normal excitation following membrane depolarization, spontaneous traveling calcium wave, resting cell with sparse, and localized calcium sparks, see Figure (b). Spontaneous local Ca2+ release occurs in the 3DVe-cell when the Ca2+ sensitivity Kposs is high, i.e. RyR clusters have a high probability of opening, even when the Ca2+ concentration is low. To evoke calcium waves in the 3DVe-cell we use a low calcium sensitivity to avoid spontaneous initiation, and a high (8pA) iCa. The evoked calcium waves propagate without decrement, and annihilate on collision, see Figure (c)(d). Since nucleus does not actively participate in the CICR and has a lower diffusion coefficient compared to cytoplasm, such as obstacle allows the same wave to return on other side of the nucleus, can give rise to an intracellular micro re-entry [3]. The 3DVe-cell is used to illustrate how stochastic properties of Ca2+ sparks can lead to complex spatio-temporal intracellular wave processes and allows the incorporation of spatial data sets (protein distribution) into the geometry.
Life Sciences 2007 (2007) Proc Life Sciences, PC520
Poster Communications: Stochastic intracellular calcium dynamics of ventricular myocytes: A simulation study
P. Li1, A. V. Holden1
1. Computataional Biology Laboratory, Institute of System and Membrane Biology, University of Leeds, Leeds, United Kingdom.
View other abstracts by:
Figure (a) the 3D geometry of the virtual cardiac myocyte. (b) Pseudo line scans of Ca2+ concentration on 50μm space (with transverse spacing of RyRs) for 1 second under different conditions. (c) 3D simulation of solitary wave initiated in one end. (d) Stimulus applied at both ends of the cell with iCa = 5.0 Kposs= 800μM waves fail to propagate due to relatively small release current iCa.
Where applicable, experiments conform with Society ethical requirements.