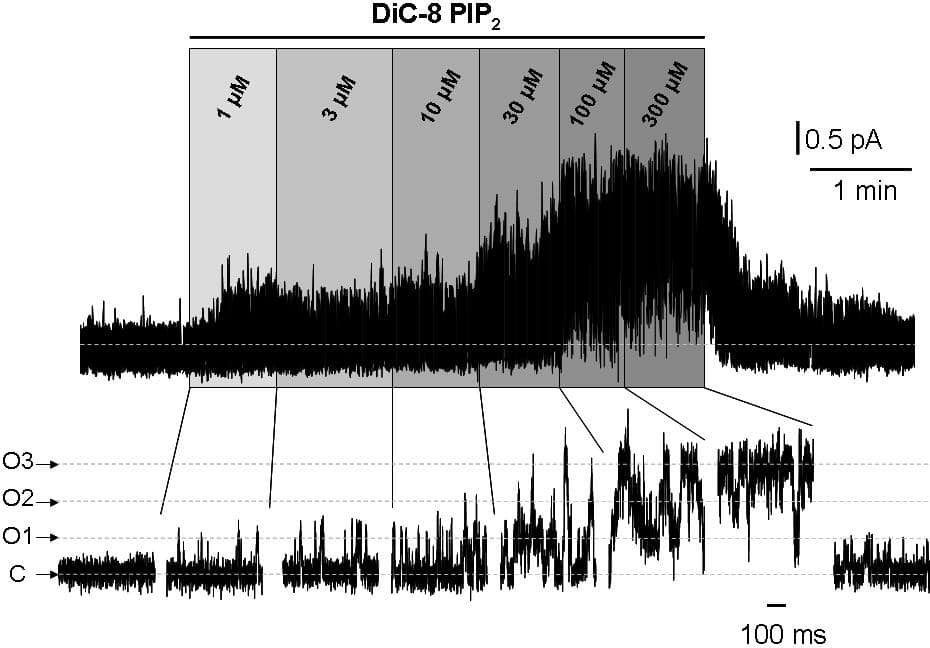
M-type potassium channels are low-threshold voltage-gated K+ composed principally of Kv7.2 and Kv7.3 subunits (1) in stoichiometrically equal proportions (2). Channel opening depends on the presence of PIP2 in the adjacent cell membrane, and close when PIP2 is depleted by (e.g.) inducing its hydrolysis with a muscarinic acetylcholine receptor (mAChR) agonist (3). Previous work (4) has shown that expressed homomeric Kv7.3 channels are ~100-fold more sensitive to the water-soluble PIP2 analogue, dioctanoyl-PIP2 (DiC8-PIP2) than homomeric Kv7.2 channels (EC50 2.6 and 205 µM respectively), and that co-expressed Kv7.2+7.3 channels had an intermediate sensitivity. In the present experiments we have further explored the concentration dependence for PIP2 activation of Kv7.2/7.3 channels, using Chinese Hamster Ovary (CHO) cells stably transfected with concatenated Kv7.2 and Kv7.3 cDNAs, to fix the channel stoichiometry at 2:2 and so replicate native M-channels (2). DiC8- PIP2 was applied in incremental concentrations from 1 to 300 µM to isolated inside-out membrane patches held at -15 mV via a fast microperfusion technique (Fig.1). Current-voltage curves recorded in cell-attached configuration before excision yielded a single-channel conductance of 9.2 ± 0.1 pS (mean ± SEM; n = 6). Mean single channel currents at -15 mV after excision were 0.55 ± 0.02 pA (n = 11). Plots of open probability (nPo) against DiC8-PIP2 concentration could be best fitted using a model scheme in which channels could bind up to 4 PIP2 molecules, with two ‘high-affinity’ sites (to Kv7.3) and two low-affinity sites (to Kv7.2). This yielded a two-component activation curve with (component 1) nPo(max1) = 0.34 , Km1 = 0.83 µM, and (component 2) nPo(max2) = 1.02, Km2 = 73 µM; nH1 and nH2 were constrained to be the same (1.76) during fitting. This implies that M-channels may open after 1 or 2 PIP2 molecules are bound but that full opening requires 4 molecules. This accords with data on PIP2-activated Kir3.4 channels (5). Partial opening at low PIP2 concentrations may also explain why maximal activation of mAChRs in sympathetic neurons produces < 100% inhibition of M-current. Thus, the Kv7.2/7.3 activity we recorded in cell-attached mode would correspond to a diC8 concentration of 12.7 µM, so ~90% reduction of PIP2 by mAChR stimulation would still leave around 15-20% M-current, in line with previous observations.
University of Manchester (2010) Proc Physiol Soc 19, PC134
Poster Communications: Stoichiometry of phosphatidylinositol-4,5-bisphosphate (PIP2) activation of expressed M-type potassium channels
V. S. Telezhkin1, D. A. Brown1, A. J. Gibb1
1. Neurosciences, Physiology & Pharmacology, University College London, London, United Kingdom.
View other abstracts by:
Effect of increasing concentrations of DiC8- PIP2 applied to an excised inside-out patch from a CHO cell expressing three M-type Kv7.2/7.3 channels, held at -15 mV.
Where applicable, experiments conform with Society ethical requirements.