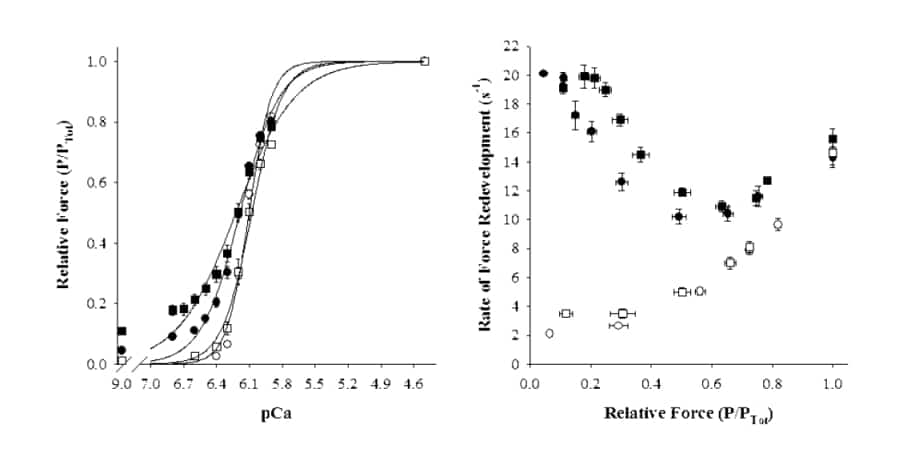
Activation of contraction in striated muscle appears to arise from positive cooperative interactions in the binding of Ca2 and myosin cross-bridges to the thin filament, such that activation of a given segment of thin filament facilitates the activation of neighboring segments. Evidence of cooperative interactions in striated muscle has been inferred from the steepness and biphasic form of the force-pCa relationship, which is steeper at low levels of Ca2 than at high, presumably due to greater intermolecular cooperation at forces less than half-maximal. Furthermore, the mechanisms of cooperative activation appear to differ between skeletal and cardiac muscle, since force-pCa relationships are generally steeper in fast-twitch skeletal muscle fibers. Expression of muscle-specific thin filament proteins (e.g., troponin C) may account for this effect by modulating the responsiveness of the thin filament to strong-binding cross-bridges.To examine possible contributions of troponin C (TnC) isoforms to cooperative activation of the thin filament, rabbit (humanely killed) skinned single psoas fibers were treated to partially extract endogenous skeletal TnC, reconstituted with exogenously added cardiac TnC, and subsequently activated in the presence or absence of a strong-binding, non-force-generating derivative of myosin subfragment 1 (NEM-S1). Relative to control fibers containing endogenous skeletal TnC, substitution of cardiac TnC reduced the steepness of the force-pCa relationship, indicating a decrease in the apparent cooperativity of activation, but had no effect on either the Ca2 sensitivity of force or the rate of force redevelopment during maximal activation. In the presence of NEM-S1, skinned fibers containing cardiac TnC exhibited greater increases in Ca2 -independent force and Ca2 sensitivity of force than fibers containing skeletal TnC. Furthermore, NEMS1 treatment accelerated the rate of force redevelopment at intermediate levels of activation to a greater degree in fibers containing cardiac TnC, but had no effect on the kinetics of force development in maximally activated preparations.These findings indicate that muscle-specific isoforms of TnC contribute to cooperative activation at submaximal [Ca2 ] by modulating thin filament responsiveness to strong-binding cross-bridges.
University of Glasgow (2004) J Physiol 557P, C5
Communications: Substitution of cardiac TnC into rabbit skeletal muscle fibers increases thin filament responsiveness to strong-binding cross-bridges
J.R. Patel, D.P. Fitzsimons and R.L. Moss
Physiology, University of Wisconsin Medical School, Madison, WI, USA
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.