Background: Sympathetic nervous stimulation (SNS) exerts critical effects on cardiac electrophysiology. Several reports have investigated whether SNS suppresses or promotes the onset of ventricular fibrillation (VF). However, is not known whether SNS alters the dynamics of VF once it is initiated. Recent optical mapping studies have revealed a wide spectrum of mechanisms that drive and sustain VF. Fibrillation mechanisms have important consequences for treatment options, for example whether targeted ablation or pharmacological treatment is optimal for preventing recurrence in VF survivors.
Methods and Results: The epicardial ventricular surface of innervated guinea pig hearts (n=8) were optically mapped at high resolution using voltage dye Rh-237. VF was induced by a dynamic S1 pacing protocol with incrementally shortening cycle length, starting at 170ms. VF was induced with and without bilateral SNS by direct stimulation of efferent sympathetic nerves in the spinal cord.
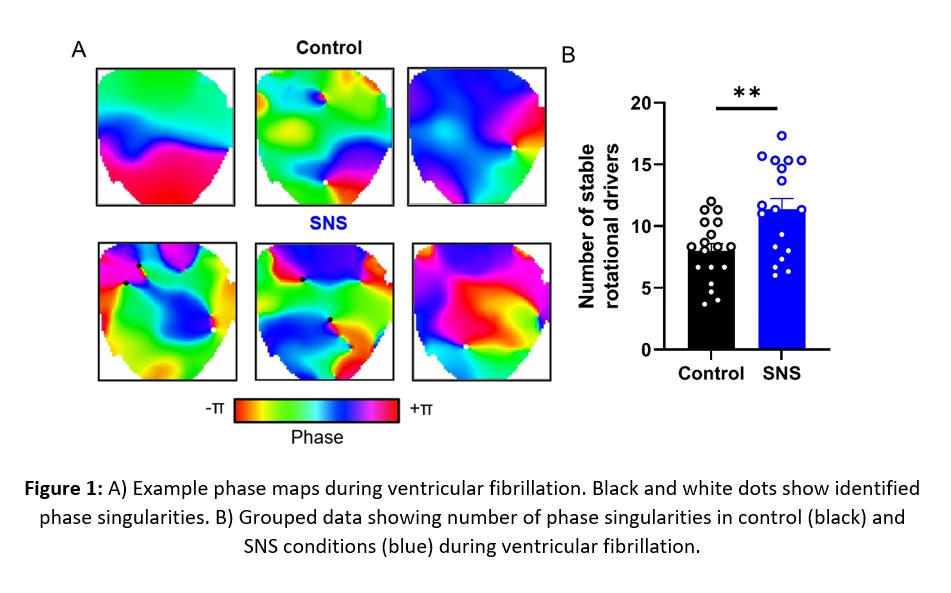
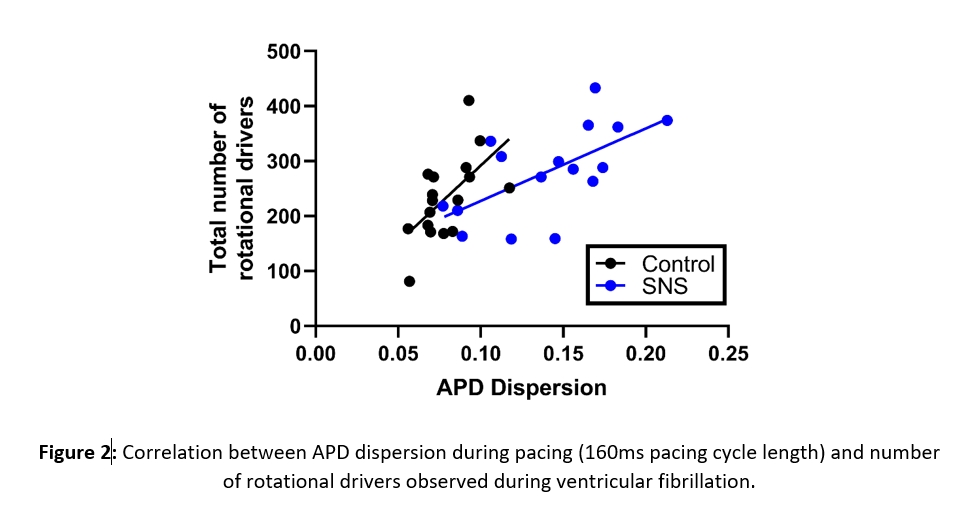
SNS shortened action potential duration, increased dispersion of repolarisation, and increased conduction velocity. Rotational activity during VF was quantified by the detection and tracking of phase singularities (PS). During SNS, the incidence of stable PSs (>2 rotations) increased by 51±13% (P<0.01). SNS also increased the time a stable PS was present during VF from 32±3% to 41±2% (P<0.01). Although SNS increased the number of PSs, it did not alter the dynamics of specific PSs such as the number of rotations, lifetime, or spatial spread. In control and SNS conditions, PSs survived for 275±30ms and 310±44ms respectively.
Conclusion: SNS increases the number of transient rotational drivers during VF. Our findings suggest SNS favours multiple stable wavelets driving fibrillation in cardiac tissue, rather than the presence of one ‘Mother Wave’. With modulation of SNS common in several pathophysiological states and treatment options, these results may hold important consequences for the clinical treatment of VF.