Heart Failure with preserved Ejection Fraction (HFpEF) is a cardiovascular disease characterized by diastolic dysfunction and microvascular rarefaction. Affecting more women than men, its main risk factors include advanced age and comorbidities like obesity, type 2 diabetes, and renal dysfunction. Shear stress (SS) homeostasis, i.e., maintenance of SS value upon hemodynamic change, contributes to vascular network structural and functional efficiency. It is achieved through SS sensing by endothelial cells (EC), a process involving EC planar cellular polarity (PCP). We hypothesized that systemic alteration of SS sensing and SS homeostasis disruption are involved in HFpEF, and associated with architectural impairment of the coronary microvasculature.
Using a mouse HFpEF model, the aim of this study was to determine the systolic (SSsys) and diastolic (SSdia) SS of the carotid, the carotid EC orientation and polarity, and the coronary capillary network density and connectivity.
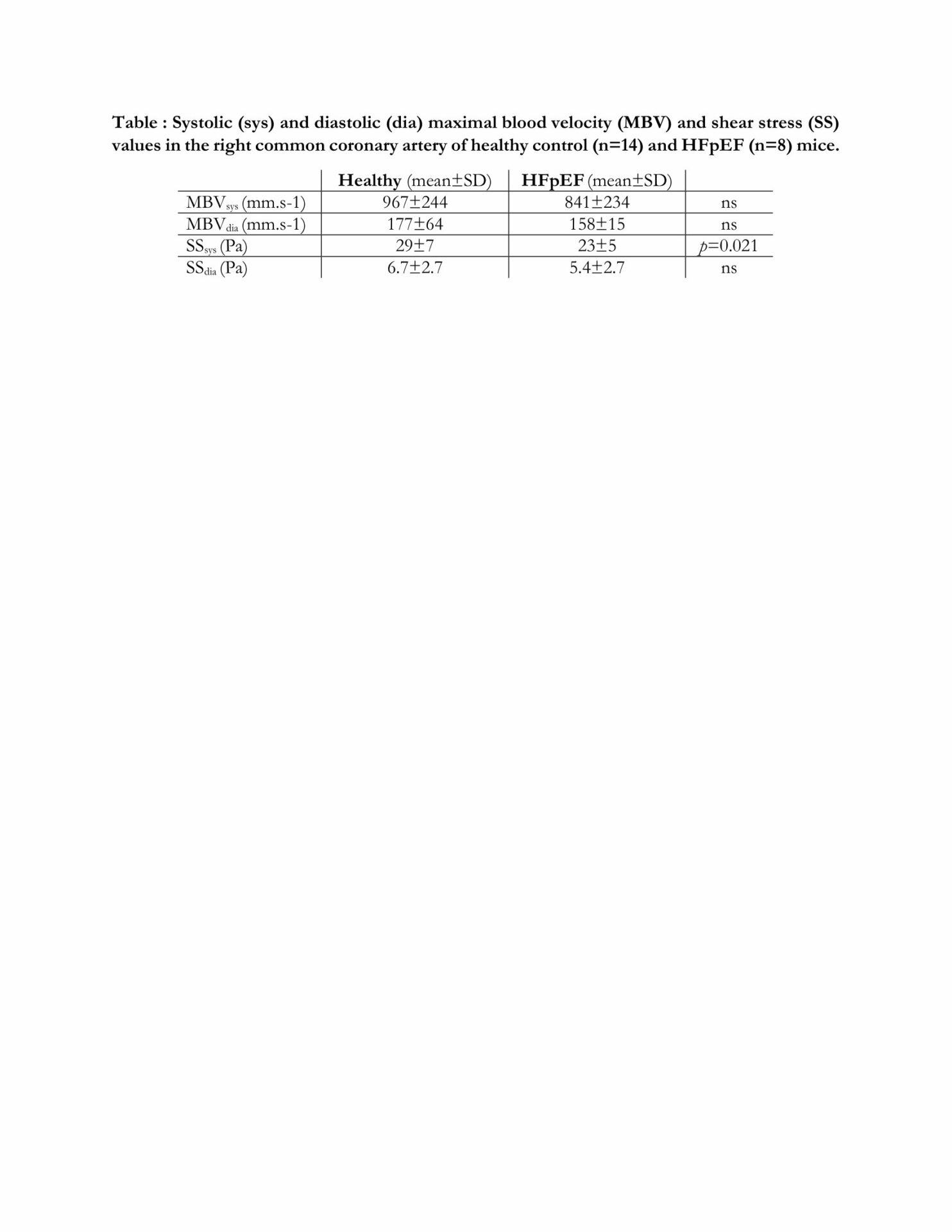
All procedures were done accorded with current national and European legislation, and agreed by the local ethical committee. 14-week-old female C57BL/Ks mice, a genetic background predisposing to renal dysfunction, and deficient for leptin receptors, inducing obesity and type 2 diabetes, were used as HFpEF model. 8-week-old C57BL/6J mice, lacking HFpEF risk factors, were used as healthy controls with 50% sex ratio, since no difference were found between sex for the studied parameters. SSsys and SSdia were calculated from the vascular diameter and maximal blood velocity (MBV) measured on the right common coronary artery (RCCA) by ultrasound imaging on anesthetized mice. After sacrifice, RCCA EC were labelled for the nucleus (DAPI) and the Golgi apparatus (Golph4) and imaged by confocal microscopy. EC orientation was measured as the angle of the nucleus-Golgi vector with blood flow direction, and classified as dromic (0°-60°), lateral (60°-120°), or antidromic (120°-180°). EC polarity was defined as nucleus elongation (major/minor axis ratio). Volumic (per mm3) vascular density (VD), segment number (SN), node number (NN), and total capillary length (TCL) of the left ventricle capillary network were calculated on processed light-sheet 3D microscopy images of lectin-labeled optically cleared hearts. Quantitative data are expressed as mean±standard deviation and compared using Student t test. EC orientation angle distributions were compared by chi2 test. Results were considered statistically significant for p < 0,05, either non-significant (ns).
Compared to healthy mice (n=14), HFpEF mice (n=8) showed no significant changes in systolic and diastolic MBV nor SSdia, but significant SSsys decrease (Table). In healthy (566 cells, 14 mice) vs HFpEF (243 cells, 7 mice) RCCA, EC orientation was 23 vs 33 % dromic, 22 vs 27 % lateral, and 55 vs 40 % antidromic, respectively, and the distribution statistically different (p=0.0006), whereas EC nucleus elongation ratio was 1.83±0.2 vs1.97±0.2 (ns). In healthy (n=14) vs HFpEF mice (n=7), VD was 46.6±9 vs 39.6±6% (p = 0.048), SN was 495,000±92,000 vs 402,000±68,000 (p = 0.019), NN was 265,000±49,000 vs 220,000±39,000 (p = 0.039), and TCL was 10±1.3 vs 8.3±1.3m (p = 0.008), respectively.
In conclusion, HFpEF mice exhibited systolic SS homeostasis disruption associated with EC PCP alteration and coronary capillary network pattern impairment.