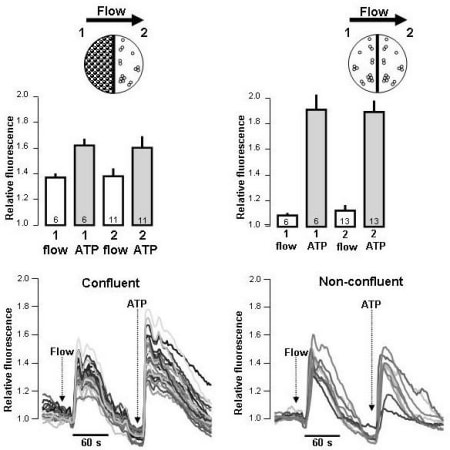
Cultured renal epithelial cells sense changes in fluid flow by their primary cilia (1). Freshly isolated renal tubules also respond changes in fluid flow by transient increases in the intracellular Ca2+ concentration, [Ca2+]i. The signal transduction pathway is, however, not well defined. Mechanical stimulation in general is known to promote release of nucleotides (ATP/UTP) and triggers auto- and paracrine activation of P2 receptors in renal epithelia. Recently, we showed that the flow-induced [Ca2+]i response in freshly isolated medullary thick ascending limb (mTAL) involves mechanically stimulated nucleotide release (2). The flow/pressure induced response was abolished by scavenging of ATP (apyrase) and by the un-selective P2-receptor antagonist (suramin). In addition, the flow/pressure induced response was strikingly lower in mice lacking the P2Y2 receptor, the predominant P2 receptor in mTAL. Our results strongly suggest that the flow-induced response is caused by release of bilateral nucleotides and subsequent activation of P2 receptors. It is, however, not known if the primary cilium is necessary for this type of response. Here, we study the influence of extracellular nucleotides on the cilium dependent flow-response and address whether ATP is released during the course of the response. We used MDCK cells in a semi-open perfusion chamber model, where the flow response is known to be absolutely cilium-dependent at low flow rates. (A) We show that the epithelial sensitivity to changing flow rates is increased at low concentrations of nucleotides that do not by themselves induce [Ca2+]i elevations. (B) Scavenging of ATP with apyrase (10U/ml) reduced the cilium-dependent flow response by 56.2%, whereas desensitisation of apical P2 receptors with ATP did not affect the response. (C) Non-ciliated MDCK cells, which do not respond to changes in fluid flow were used as reporter cells for paracrine ATP signalling. MDCK cells were grown on half coverslips and the non-confluent indicator cells were positioned on the outflow side, directly next to the confluent cells. Intriguingly, the non-ciliated cells only responded to fluid flow with confluent ciliated cells on the inflow side (fig.). This was not the case if non-confluent, non-ciliated cells were placed on the inflow side. This paracrine response was reduced by scavenging of ATP. The data allow us to conclude that flow-induced, primary cilia-dependent [Ca2+]i signalling is potentiated by stimulated nucleotide release.
University of Bristol (2008) Proc Physiol Soc 9, C5
Oral Communications: The cilium-dependent flow-response is amplified by nucleotide release in MDCK cells
H. Praetorius1, J. Leipziger1
1. Institute for Physiology and Biophysics, University of Aarhus, Aarhus, Denmark.
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.