L-type Ca2+ channels are located predominantly at the t-tubules of rat ventricular myocytes, co-localised with the Ca2+ release channels of the sarcoplasmic reticulum (Scriven et al. 2000). The mechanism by which the L-type Ca2+ channels are trafficked and targeted is unknown, although it has been suggested that the cytoskeleton may play an important role. We have, therefore, investigated the effect of the actin depolymerising agent cytochalasin-D on L-type Ca2+ channel distribution.
Wistar rats were killed humanely. Ventricular myocytes were enzymatically isolated, and then maintained in long-term (<= 8 days) culture in serum-free medium on laminin-coated coverslips (Mitcheson et al. 1996). Cultured cells were probed with an anti-calcium channel α1 subunit antibody.
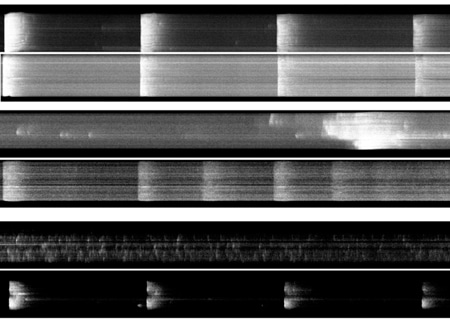
In control cells, staining the cell membrane with di-8-ANEPPS showed regular transverse staining (t-tubules) at ~2 µm intervals at 0 h; t-tubule density subsequently decreased to 44 % by 96 h. During this time the cells lost their characteristic rod-shaped morphology and staining actin with phalloidin showed disorganisation of its normal staining pattern. After fixing in 4 % (v/v) paraformaldehyde, antibody staining of the α1 subunit of the L-type Ca2+ channel, using an avidin-biotin immunocytochemical staining procedure (Maier et al. 2002), revealed a shift from a linear transverse to a punctate longitudinal staining pattern during this time, with concentration of punctate staining in the perinuclear region after 96 h. Ca2+ transients, elicited by field stimulation of fluo-3-loaded myocytes, showed loss of synchronous Ca2+ release across the cell width on stimulation and the appearance of asynchronous Ca2+ sparks (Fig. 1).
In contrast, cells cultured for 96 h in 40 µM cytochalasin-D retained 84 % of their t-tubular structure, their rod-shaped morphology, orderly actin staining, and linear transverse anti-α1 subunit antibody staining. Furthermore, synchronous Ca2+ transients could be elicited for longer by field stimulation (Fig 1). Cells incubated in the presence of 20 µM cycloheximide, a protein synthesis inhibitor, showed a similar stabilisation of morphology and function up to 48 h, suggesting that once inserted in the membrane the turnover of the α1 subunit is relatively slow.
These data show that cytochalasin-D appears to stabilise actin and cell morphology during culture. The loss of the normal α1 staining pattern in control cells, despite its slow turnover, and the accumulation of perinuclear α1 staining suggest that normal actin structure is required for anchoring and trafficking the α1 subunit.
This work was supported by the Wellcome Trust and British Heart Foundation