The rising prevalence of overweight and obesity represents a significant health concern that remains insufficiently addressed by current therapeutic options. Thermal interventions offer a potential alternative for improving metabolic health. Recent studies highlight the positive effects of both repeated heat or cold exposure on glucose homeostasis and cardiovascular health [1-4]. However, the impact of combined heat and cold therapy, with potential amplified/additive effects (or a diminished effect), on the previously observed benefits, remains largely unexplored. While the use of sauna has been shown to have beneficial effects on cardiovascular health [5], its metabolic effects remain largely unstudied.
The presented study will assess whether infrared sauna followed by cold-water immersion improves glucose homeostasis in healthy, overweight volunteers. Additional exploratory outcomes including thermophysiological, cardiovascular, metabolic and functional outcomes, as well as inflammatory markers, muscle structure, subjective perception, and mental well-being will be assessed.
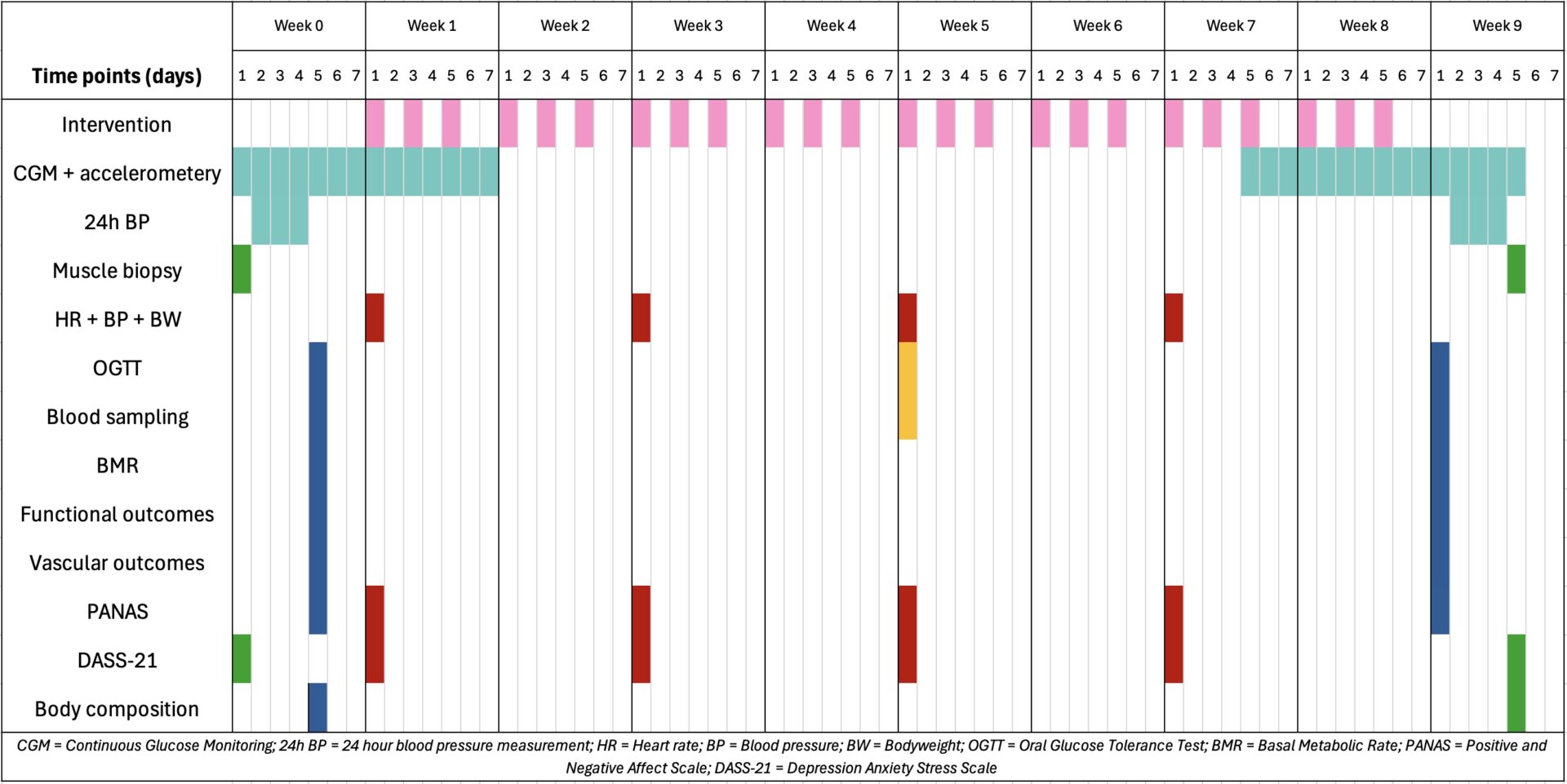
A single-arm, within-subject experimental trial will be conducted. Twenty volunteers between 40-75y with a BMI between 27.5-35 kg/m2 will be recruited. Participants will undergo the intervention three times per week for eight weeks (Fig. 1, pink boxes). After a familiarisation week, each session will consist of two 20-minute sauna exposures (70ºC) (HM-LSE-3 Professional edition, Health Mate, Belgium), followed by 2-minute cold-water immersions (±14ºC) up to the clavicula bones, with a 10-minute rest at room temperature. Three days before and after the intervention, a basal metabolic rate measurement (Maastricht Instruments, Omnical), an oral glucose tolerance test (OGTT) and vascular measurements (pulse wave analysis and velocity (Atcor Medical, SphygmoCor v9), flow mediated dilation (MyLabGamma, Esaote), retinal imaging (Topcon Corporation, Topcon TRC-NW-300) and laser doppler flowmetry (Moor Instruments, moorLAB-HEAT and moorLAB-LDF2) will be conducted (Fig. 1, blue boxes). Additionally, resting heart rate and blood pressure (Omron, M7 Intelli IT), functional outcomes (submaximal cardiopulmonary exercise testing (Lode B.V., Lode Corival CPET) and handgrip strength (Fred Sammons, Inc, JAMAR handheld dynamometer), and a questionnaire on positive and negative affect (PANAS) will be evaluated alongside blood sampling to assess plasma volume, and metabolic (lipid metabolites, renal and liver function) and inflammatory markers.
In the week before and after the intervention period, assessments on muscle structure (m. vastus lateralis biopsy), body composition (doubly-labelled water), and psychological symptoms (DASS-21) will be conducted (Fig. 1, green boxes). Midway through the intervention, an additional OGTT will be performed alongside blood sampling to assess evolution of metabolic and inflammatory markers, and plasma volume (Fig. 1, yellow boxes).
Every two weeks, total sweat loss (bodyweight change), skin (Maxim Integrated Products, iButtons) and core temperature (eCelsius Performance, Body Cap), heart rate (Polar Electro, H10), blood pressure, subjective perception (e.g. ASHRAE-55 7-point VAS), and mental well-being will be monitored during exposure (Fig. 1, red boxes). Moreover, before and after the intervention, glucose (Abbott GmbH & Co, FreeStyle Libre Pro) and physical activity (ProCare BV, Actigraph wGT3X-BT) levels will be continuously tracked over two weeks, and a 24-hour blood pressure measurement (Omron M7, CEMEX) will be conducted (Fig. 1, turquoise boxes).
Preliminary data from pilots and first participants will be presented during the conference.