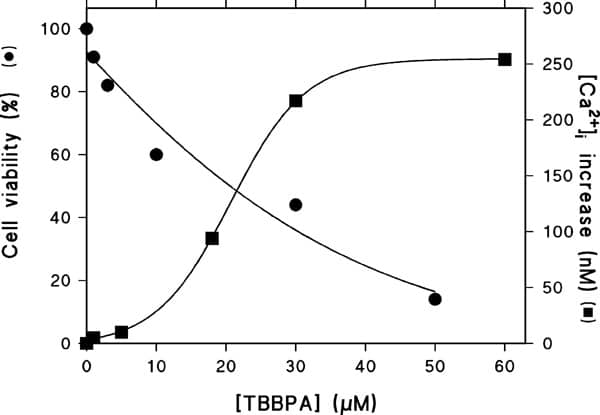
Brominated flame retardants (BFRs) are a family of compounds used at high levels in the modern world. Their purpose is to slow down the spread of fire in materials in which they are incorporated. One-third of all BFRs produced worldwide consist of tetrabromobisphenol-A (TBBPA) [1]. Popularity in the use of TBBPA in recent years has led to its high level of disposal in the environment. It is now a pollutant commonly found in sewage and air [2]. BFRs are capable of accumulating in living organisms, with some having been detected in human blood at levels of 160ng/g lipid serum [3]. Recent reports have identified TBBPA to act as an ‘endocrine disruptor’ by perturbing the activities of thyroid hormones and estrogen [4]. Our previous studies have linked some endocrine disrupting pollutants with dysregulation of Ca2+ signalling in testicular Sertoli cells, which are involved in sperm maturation and development. We and others have speculated that exposure to these pollutants, may in part, contribute to the increased incidence of male infertility currently being reported [5]. Here we present data to show that TBBPA can affect Ca2+ homeostasis and induce cell death in Sertoli cells. The TM4 mice Sertoli cell line was used in these experiments. Using Fura-2-based fluorimetry as described in [5], TBBPA was shown to cause a dose-dependent increase in intracellular [Ca2+] levels ([Ca2+]i) in TM4 cells (EC50, 22μM), with 60μM TBBPA increasing the [Ca2+]i by 250nM from 100nM to 350nM (fig.1). As TBBPA also increased [Ca2+]i in cells incubated in buffer containing low [Ca2+], this rise is likely due to mobilization from intracellular Ca2+ stores. From related work with other endocrine disrupters, we believe that this rise is due to TBBPA inhibiting SERCA Ca2+ pumps [5]. Cell viability as assessed using the MTT assay, described in [5], showed that treatment of TM4 cells with TBBPA (0-50μM) for 18 hours, caused a dramatic increase in the degree of cell death (LC50, 20μM) (fig.1). Measurements of mitochondrial membrane potential of TBBPA-treated TM4 cells, using rhodamine-123, also showed that TBBPA at concentrations as low as 5μM could depolarise the mitochondrial membrane, by opening the permeability transient pore (PTP). Our data indicate that TBBPA can cause abnormally high levels of intracellular [Ca2+] within Sertoli cells, which could induce ‘Ca2+ overload’ of the mitochondria, leading to the opening of the PTP. This in turn could cause the release of pro-apoptotic factors from the mitochondria leading to the observed decrease in cell viability.
Life Sciences 2007 (2007) Proc Life Sciences, PC271
Poster Communications: The effect of tetrabromobisphenol-A, a brominated flame retardant, on Ca2+ signalling and cell viability in Sertoli cells
P. F. Lai1, O. A. Ogunbayo1, T. J. Connolly1, F. Michelangeli1
1. Biosciences, university of Birmingham, Birmingham, United Kingdom.
View other abstracts by:
The effects of TBBPA on cell viability (●) and increases in intracellular Ca2+ levels (■) within TM4 Sertoli cells
Where applicable, experiments conform with Society ethical requirements.