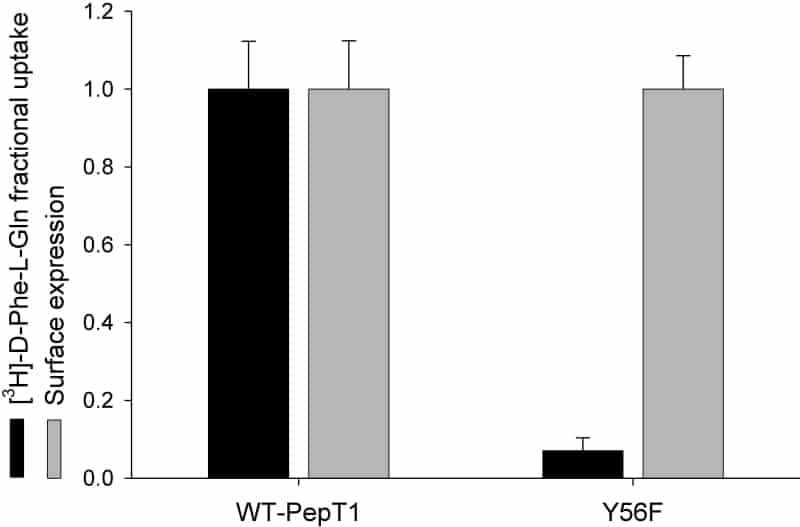
PepT1 mediates the intestinal absorption and renal re-absorption of di- and tri-peptides and a great number of therapeutically active compounds (Daniel & Kottra, 2004). It has previously been proposed that a conserved histidine (H57) in the second transmembrane domain (TMD2) of the transporter is protonated and binds the carboxy terminus of the substrate (Bailey et al. 2000). Tyrosine56 (Y56) is the adjacent residue to this histidine and has been proposed to stabilize the positive charge on the protonated histidine (Chen et al. 2000). Here we report the findings of further testing the role of Y56 by site-directed mutagenesis. Tyrosine56 was mutated to a phenylalanine (giving Y56F-PepT1), and expressed in Xenopus oocytes. Both membrane expression and the transport properties of Y56F-PepT1 were determined by luminometry and uptake of 0.4μM [3H]-D-Phe-L-Gln respectively using techniques previously described (Panitsas et al. 2006). Data are means ± SEM of n oocyte preparations. As can be seen in Fig. 1, the uptake of D-Phe-L-Gln into oocytes expressing Y56F-PepT1 was significantly slower than for those expressing wild-type PepT1 (wt-PepT1), even when the level of protein surface expression is taken into account. Comparing the affinity for Gly-L-Gln revealed that the mutant had a greatly increased affinity for this neutral dipeptide, with a Ki at pHout 5.5 of 6 ± 1μM for Y56F-PepT1 compared with 324 ± 82μM for wt-PepT1 (n=4). This higher affinity was maintained at pHout 7.4 (5 ± 1μM and 244 ± 33μM for Y56F-PepT1 and wt-PepT1, respectively, n=3). The finding that the affinity of Y56F-PepT1 is so dramatically different from that of wt-PepT1 suggests that Y56 either directly forms part of, or exerts an influence on, the substrate binding site of PepT1. One possible explanation is that Y56 stabilises H57 residue when in its deprotonated (rather than protonated) form, and thus when this effect is removed the substrate binds more tightly. This is consistent with the reduction in the rate of transport seen with Y56F-PepT1, with the release of the substrate from the binding site after translocation becoming the slowest step in the transport cycle.
University of Manchester (2006) Proc Physiol Soc 2, PC18
Poster Communications: The functional role of tyrosine56 in the rabbit proton-peptide cotransporter, PepT1 expressed in Xenopus oocytes
Myrtani Pieri1, CAR Boyd1, Pat Bailey2, David Meredith1
1. Physiology, Anatomy & Genetics, University of Oxford, Oxford, United Kingdom. 2. School of Chemistry, University of Manchester, Manchester, United Kingdom.
View other abstracts by:
Figure 1. Normalised uptake of D-Phe-L-Gln and transporter surface expression in Xenopus oocytes expressing wild-type PepT1 (wt-PepT1) or Y56F-PepT1. The Y56F-PepT1 uptake is reduced to <10% of that of the wild-type (p<0.01 Student’s paired t test n=3).
Where applicable, experiments conform with Society ethical requirements.