G protein-gated inwardly rectifying K+ channels expressed in cardiac and neuronal cells (GIRK or Kir3.x family) are activated by interaction with βλ dimers released from heterotrimeric G proteins upon stimulation of appropriate receptors. There is a controversy about whether activation in principle is limited to βλ-dimers released from pertussis toxin (Ptx)-sensitive G proteins. This would suggest that the α-subunit of heterotrimeric G proteins confers specificity to Gβλ signalling, as has been proposed recently (Leaney et al. 2000). More recently overexpressed β1-adrenergic receptors have been shown to couple to GIRK channels via Gs in atrial myocytes (Wellner-Kienitz et al. 2001).
The cardiac type of GIRK channel composed of Kir3.1/Kir3.4 was expressed by transient transfection in CHO and HEK293 cells and activation of GIRK current by different co-expressed G protein-coupled receptors was studied to address the question of whether the type of G protein confers specificity to signalling via Gβλ.
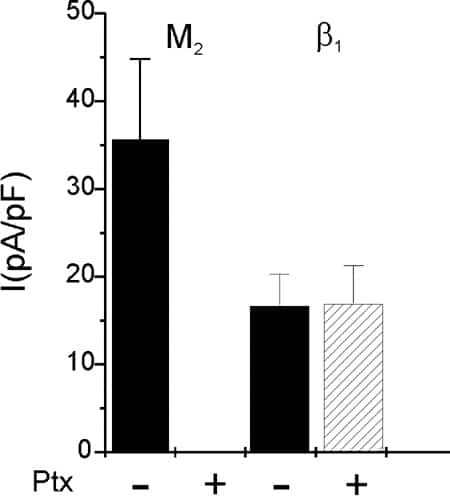
Robust activation of current was observed upon stimulation of receptors coupling to Gi/o (M2-muscarinic, A1-purinergic, A3-purinergic, S1P2, S1P3 sphingolipid) that was completely abolished by Ptx. Via GS coupled receptors (β1-adrenergic, A2 purinergic, H2 histaminergic) currents with about 50 % less density could be activated that were insensitive to Ptx treatment (Fig. 1). Comparable results were obtained in both CHO and HEK293 cells.
These data indicate that G protein α subunits do not play a central role in determining receptor specificity of coupling to G protein-gated inwardly rectifying K+ channels.
This work was supported by the Deutsche Forschungsgemeinschaft (Po212/11-1).