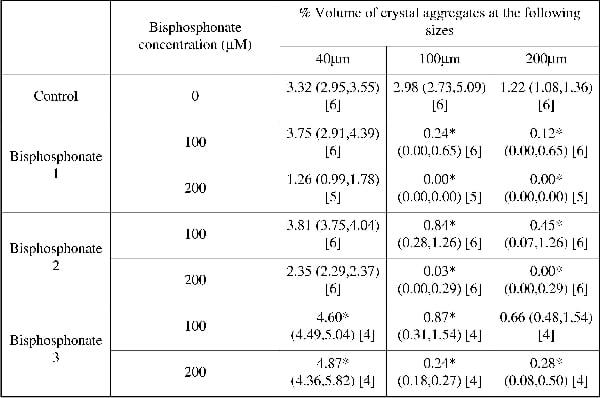
Calcium oxalate (CaOx) is the most common constituent of urinary stones. These in vitro experiments have studied the physical chemistry of CaOx formation, and analysed the inhibitory effect of bisphosphonates on their aggregation in urine. Calculus growth increases most rapidly by crystal aggregation rather than individual crystal growth; our hypothesis is that stone formation is decreased dramatically if aggregation could be inhibited or even slowed. CaOx crystals were formed by mixing equal volumes of two solutions, ‘A’ (containing calcium) and ‘B’ (containing oxalate). The remaining salts in these solutions combined to form a recognised ‘artificial urine’ (Robertson & Scurr, 1986). Human urine was not used due to its considerable chemical variability. The solutions were mixed at 37°C and the crystals were formed by the mixed suspension mixed product removal method. The outflow of the mixing chamber was pumped into a Mastersizer (Malvern Instruments, UK) to measure the number and size of particles using low angle laser scattering. Several bisphosphonates (potential inhibitors of crystal aggregation) were added to solution ‘B’ and the change to the percentage volume occupancy of crystals at sizes 40, 100 and 200 µm was recorded. Data are median values (25%, 75% interquartiles) and changes to volume occupancy from control by bisphosphonates tested by Mann-Whitney U test; significance was accepted at p<0.05. Table 1 shows the volume occupancy of CaOx crystals of different sizes in control conditions and after addition of three different bisphosphonates at 100 and 200 µM. Bisphosphonates 1 and 2 reduced significantly the number of 100 and 200 µm crystals at both concentrations. The third bisphosphonate also reduced the larger crystal numbers at 200 µM. Bisphosphonates are currently used to treat bone disease and are excreted in the urine. Furthermore, they are resistant to alkaline phosphatase and acid conditions, unlike other inhibitors of urinary crystal aggregation, such as polyphosphates. The ability of these agents to prevent CaOx aggregation to sizes of ≥100 µm will therefore limit their tendency to block nephrons and thus have potential to protect against urinary stone formation.
University College London 2006 (2006) Proc Physiol Soc 3, PC17
Poster Communications: The inhibitory effect of bisphosphonates on calcium oxalate crystal formation in vitro
Sian Elizabeth Allen1, Simon Choong1, Christopher Fry1, William G Robertson1
1. Institute of Urology & Nephrology, University College London, London, United Kingdom.
View other abstracts by:
Table 1. Volume occupancy of CaOx crystals of different sizes in artificial urine containing three different bisphosphonates
Where applicable, experiments conform with Society ethical requirements.