The N-blocked amino acid N-acetyl-L-phenylalanine (Ac-Phe) has previously been shown to be a competitive inhibitor of the peptide transporter PepT1 with a Ki of 1.8 mM (Guha et al. 1999; Meredith et al. 2000). However, Ac-Phe would not be predicted to bind in the same configuration as other substrates by our current template model of the PepT1 substrate-binding site (Bailey et al. 2000) and therefore was not expected to be a substrate. To test this we have used a trans-stimulation of efflux assay to ascertain whether Ac-Phe is a translocated substrate.
PepT1-expressing Xenopus laevis oocytes were injected with 4.6 nl 3H-D-Phe-L-Gln (56 MBq ml-1). Efflux studies were performed by placing five oocytes into 100 µl of medium (95 mM NaCl, 2 mM KCl, 1 mM CaCl2, 20 mM Tris/Mes; pH 5.5) plus the appropriate test compound (at the Ki or Km concentration value) for 90 min. After this time, an aliquot of the incubation medium was taken and the oocytes were washed in ice-cold medium and lysed with 2 % (w/v) SDS. The oocytes and the aliquot of medium were scintillation counted. All data are means ± S.E.M., with n = 5 oocytes per data point.
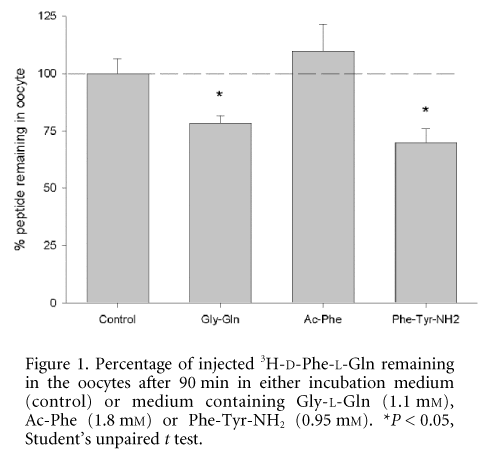
As can be seen in Fig. 1, the control compound Gly-L-Gln trans-stimulated the efflux of D-Phe-L-Gln with 78.6 ± 3.1 % of the peptide remaining in the oocyte after 90 min, whereas for Ac-Phe there was no trans-stimulation (109.6 ± 11.7 % remaining). In support of this the amount of radiolabelled peptide in the incubation medium was increased 7.8- and 1.2-fold, respectively. In comparison, the C-terminal blocked dipeptide L-phenylalanyl-L-tyrosine-amide (Phe-Tyr-NH2) stimulated a similar level of trans-stimulation to Gly-L-Gln, consistent with electrophysiological evidence for its translocation into PepT1-expressing oocytes (Beattie & Boyd, 2000). No efflux was seen in non-injected oocytes under the same conditions.
These data, when taken with the previously available evidence, are consistent with Ac-Phe being a non-translocated competitive inhibitor for PepT1. This finding validates the prediction of the template binding model that Ac-Phe is binding in an atypical manner, and hence should not be translocated.
We thank M.A. Hediger for the PepT1 clone and The Wellcome Trust for their generous support.
All procedures accord with current UK legislation.