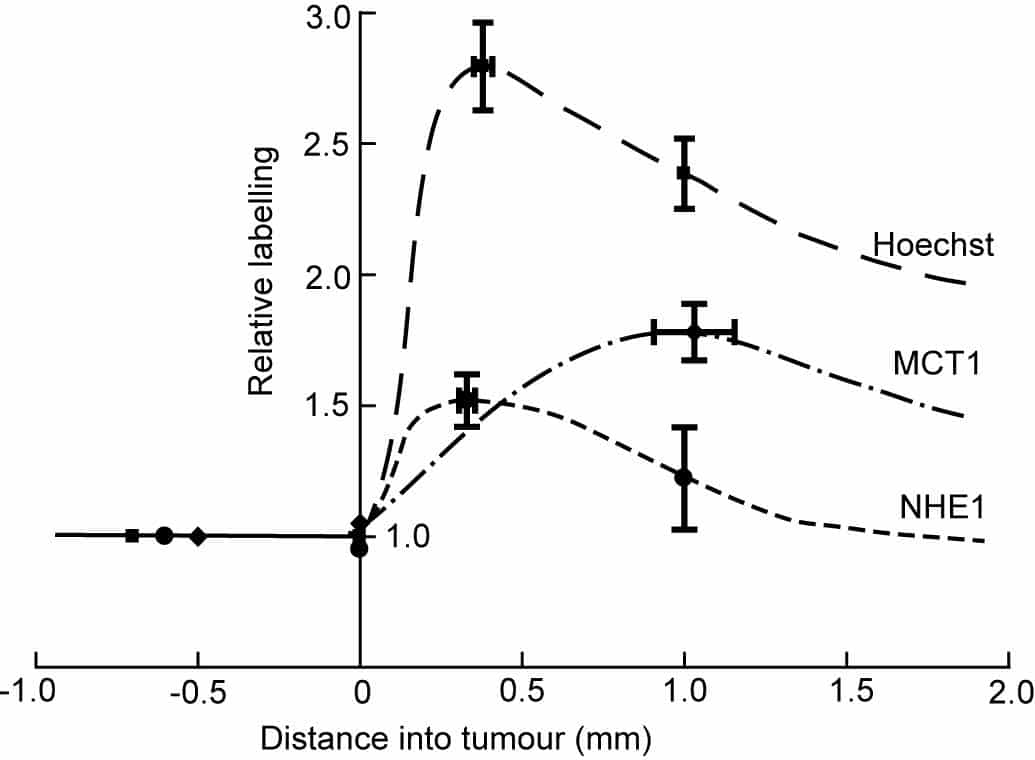
For a systemically administered drug to act preferentially on a tumour, the drug must recognize some characteristic of the tumour, such as rapid replication of DNA. A more specific characteristic of tumour cells is the unusual gradient of pH across their plasma membranes. Most normal cells have an intracellular pH (pHi) of 7.0 – 7.2 and an extracellular pH of 7.4. In tumour cells, the gradient of H+ activity is reversed: pHi is typically about 7.2 while extracellular pH is 6.5 – 7.1 (1, 2). Because of this gradient, drugs that are weak acids tend to accumulate in tumour cells, and pH-dependent drug carrier molecules can be constructed (3). The ubiquitous Na+/H+ exchanger, NHE1, is upregulated in invadopodia of cancer cells in culture (4) and some tumours are known to express the isoforms MCT1 and MCT4 of the H+-lactate cotransporter. We have looked at the spatial distributions of these H+ transporters in C6 gliomas in rat brains. Procedures conformed to European Council Directive 86/609/EEC (5). Brain sections were immunolabelled and tile-scanned. NHE1 labelling was greatest at the growing tumour rim. On average, the peak was 1.52 times higher than in extratumoural tissue (SEM = 0.10, 11 gliomas, P = 0.0004 by Student’s two-tailed t test) at an average distance of 0.33 ± 0.02 mm from the edge, as defined by the intensity of the nuclear stain Hoechst 33342. MCT1 also peaked in the rim (1.78 ± 0.11 fold) but the peak was significantly further into the glioma (1.03 ± 0.12 mm from the edge, P < 0.0001 for the difference; Fig. 1). Mean labelling of MCT1 in viable parts of the gliomas was higher than mean labelling outside by a factor of 1.33 ± 0.10. We also looked at labelling of MCT4 and of the carbonic anhydrase CAIX. Both are upregulated by hypoxia, as may occur in tumours. Labelling of MCT4 and CAIX was present in tissue inside and outside the gliomas, but showed no systematic increase in the rim. This result makes it unlikely that the peaks of Hoechst, NHE1 and MCT1 were artefacts due to uneven tissue shrinkage. There were localized increases in labelling of MCT4 and CAIX within the gliomas which were highly correlated in space. We conclude that the growing rim of a C6 glioma is spatially organized on a scale of hundreds of microns, with a concentration of NHE1 near the growing border. We suggest that in the periphery, where oxygen is available, MCT1s take up lactate and H+ released from hypoxic areas. Since these H+ ions are stoichiometrically required for oxidative phosphorylation, it is not clear where the H+ required for NHE1 come from.
University of Manchester (2010) Proc Physiol Soc 19, C20
Oral Communications: The Na+/H+ exchanger NHE1 and the lactate-H+ cotransporter MCT1 are spatially organized in C6 gliomas in rat brain.
J. A. Coles1,3, E. Grillon2,3, R. Farion2,3, K. Fablet2,3, M. De Waard2,3, M. Tse4, M. Donowitz4, C. Segebarth2,3, C. Rémy2,3
1. SIPBS, Strathclyde University, Glasgow, United Kingdom. 2. INSERM U836, Grenoble, France. 3. Universite Joseph Fourier, Grenoble, France. 4. Department of Physiology and Medicine, Johns Hopkins University, Baltimore, Maryland, United States.
View other abstracts by:
Figure 1. NHE1 and MCT1 peak near the tumour rim. Labelling relative to extratumoural tissue was measured at the peak intensity and (for Hoechst and anti-NHE1) at 1 mm into the tumour. Mean values for the 11 tumours have been plotted and joined by freehand curves. Bars show SEMs.
Where applicable, experiments conform with Society ethical requirements.