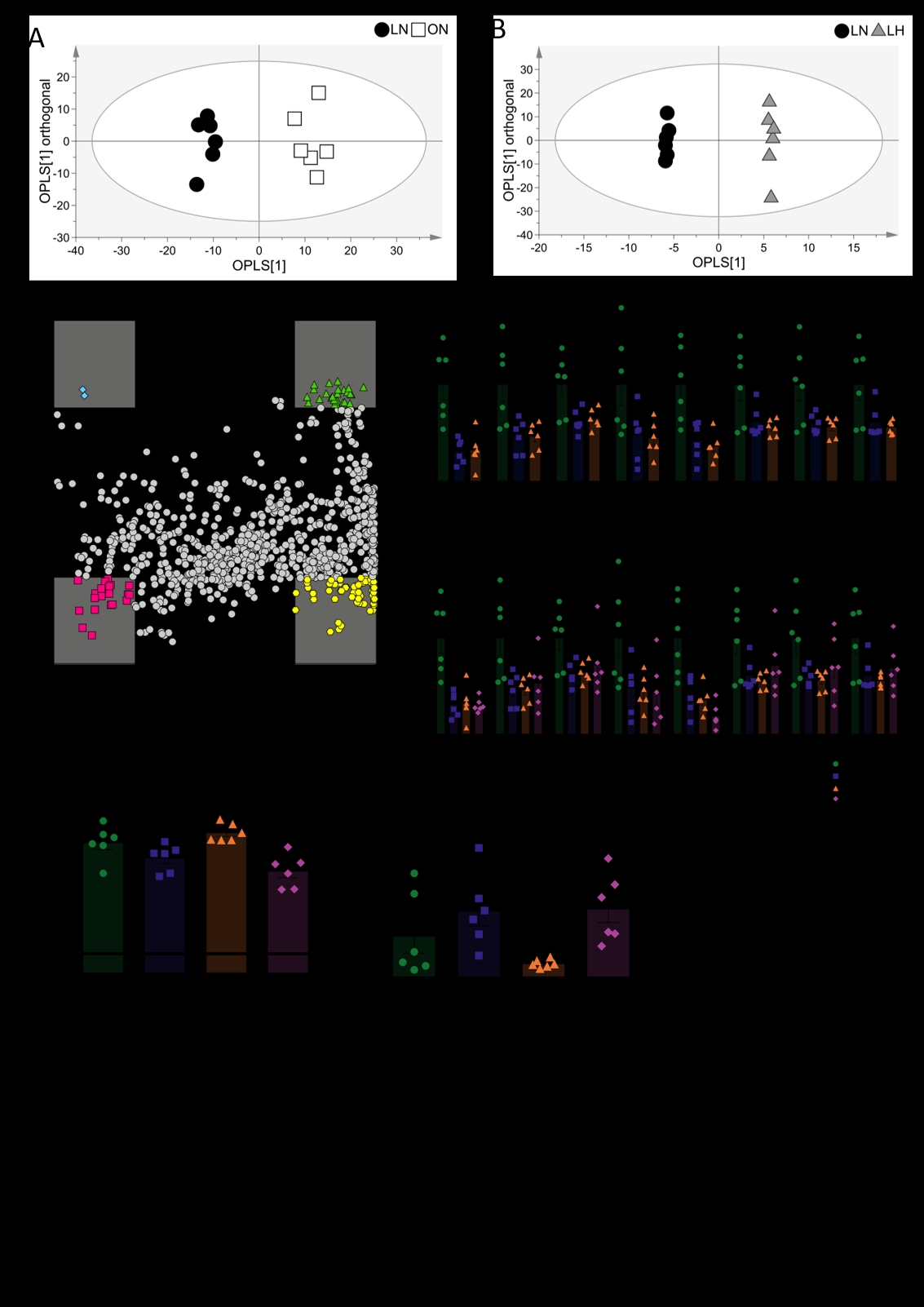
Hypoxia is associated with the aetiology of obesity. For instance, expanded adipose tissue experiences a lower oxygen tension and shows altered metabolism as a result [1]. However, in addition to adipose, the liver enlarges during obesity in association with ectopic fat storage [2]. Moreover, systemic inflammation leads to localised hypoxia as activated immune cells increase oxygen consumption and therefore deplete tissue oxygen tension [3]. We therefore sought to investigate the metabolic overlap between hypoxia and obesity in rat liver to determine whether hypoxia signalling plays a role in the development of obesity-related hepatic conditions, such as non-alcoholic liver disease. All experiments were carried out in accordance with the UK Home Office, Animals (Scientific Procedures) Act (1986) and were reviewed by the University of Cambridge Animal Welfare and Ethical Research Committee. Following 7-day baseline measurements in controlled environmental conditions (22°C, 55% humidity, 12h photoperiod), female Zucker obese (ZUC-Leprfa/fa) and lean (ZUC-Leprfa/+) rats were either exposed to two days of normobaric hypoxia (10.3 ± 0.2% O2; hypoxia groups) or maintained in the same environmental conditions (normoxia groups; n = 6 per group). At the end of the two-day exposure, the left lateral lobe of the liver was harvested and snap-frozen for metabolic analysis. Liquid chromatography-mass spectrometry (LC-MS) was utilised to carry out open-profile lipidomics on the liver. Using orthogonal partial least squares-discriminate analysis, the obese/normoxic lipidome was shown to differ significantly to that of the lean/normoxic lipidome (R2X(cum) = 81.4%, Q2(cum) = 84.9%, p<0.0001 ). Likewise, the lean/hypoxic lipidome differed to the lean/normoxic lipidome (R2X(cum) = 85.1%, Q2(cum) = 81.3%). These models were used to generate a shared and unique structures plot, with species within the ±0.5 region on either axis being of interest as shared or unique to obesity and/or hypoxia. Of these species of interest, a series of sphingomyelins (chain length 24:0 – 41:1) were found at lower levels in both the hypoxic and obese liver (p<0.05, one-way ANOVA with Benjamini-Hochberg false discovery rate correction, Tukey’s post hoc test for multiple comparisons). These species remained at lower levels (compared to lean/normoxic controls) when the insults were combined in the obese/hypoxic liver. Using LC-MS, the acyl-carnitine complement of the liver was also assayed. The obese/hypoxic liver had higher levels of long-chain acyl-carnitines (≥13 carbons) compared with the obese/normoxic liver, alongside lower levels of short-chain acyl-carnitines (<6 carbons). This suggests that during hypoxia, fatty acid oxidation decreases in the obese liver [4], showing that the obese liver remains metabolically flexible to respond to hypoxic conditions by promoting less oxygen-expensive pathways. Taken together, these results highlight that there are metabolic signatures common to the obese and hypoxic liver. However, the obese liver can still respond metabolically to hypoxic stimuli despite potential lipotoxicity and insulin resistance concurrent with obesity development [5].
Future Physiology 2021 (Virutal) (2021) Proc Physiol Soc 47, OC16
Oral Communications: The obese rat liver displays overlapping lipid signatures to the hypoxic liver, yet retains metabolic flexibility to respond to acute hypoxic stress
Alice P. Sowton1, 2, Fynn N. Krause2, Katie A. O'Brien1, Andrew J. Murray1, Julian L. Griffin2, 3
1 Department of Physiology, Development and Neurosceince, University of Cambridge, Cambridge, United Kingdom 2 Department of Biochemistry, University of Cambridge, Cambridge, United Kingdom 3 Section of Biomecular Medicine, Department of Metabolism, Digestion and Reproduction, Imperial College London, London, United Kingdom
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.