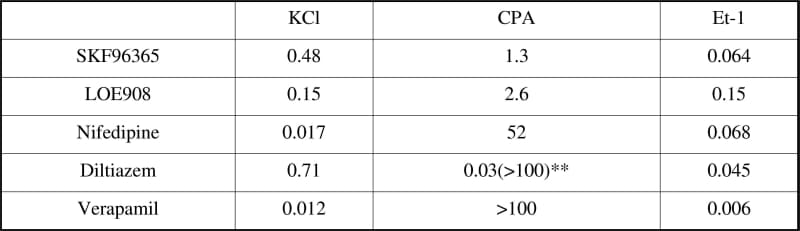
Smooth muscle cells express a variety of voltage-independent Ca2+ channels but studies on the functional role of these has been hampered by their confused pharmacological susceptibilities. Previously, we showed that nifedipine in rabbit choroid arterioles, and mibefradil in rat retina appeared to block store-, receptor- and L-type Ca2+ channels at the same equally low concentrations. Here we used a range of classical Ca2+ channel blockers to identify agents which might be useful in delineating voltage-dependent and -independent Ca2+ channels in the same arteriolar smooth muscle cells. Arteriolar fragments (15-35 μm outside diameter) were isolated by gentle trituration of the retinae removed immediately from humanely killed rats. After loading with fura-2 AM, cytosolic Ca2+ was measured in the smooth muscle layer by microfluorimetry. Ca2+ influx was measured as the rate of Ca2+ rise on adding 1 mM to the Ca2+-free medium normally superfusing the microvessels. This was performed in the presence of either: (a) 70 mM KCl (to open voltage-dependent Ca2+ channels), (b) cyclopiazonic acid (10 Mμ CPA, to stimulate store-depletion operated Ca2+ channels), or (c) endothelin-1 (10nM Et-1, to activate receptor-controlled Ca2+ influx). Concentration-effect curves were constructed for the Ca2+ channel blockers and these used to read off ID50s (Table 1). The effects of SKF96365 and LOE908 were not consistent with their specific actions on either store or receptor induced Ca2+ influxes. In this preparation store-operated channels were resistant to nifedipine in contrast to rabbit choroidal arterioles. The results with diltiazem and verapamil suggest that these cells have at least three voltage-independent Ca2+ influx pathways which can be separated pharmacologically. Two of these are store-depletion activated channels and can be separated by their differential sensitivity to diltiazem but both have poor sensitivity to verapamil. The receptor-operated component is highly sensitive to both drugs. TRP channels are thought to underlay voltage-indendent Ca2+ influx pathways in smooth muscle and a similar screening approach of known Ca2+ channel blockers could be a useful strategy to find pharmacological tools which can be used to associate functions with particular molecular entities.
University of Oxford (2005) J Physiol 568P, PC55
Poster Communications: The pharmacology of voltage-insensitive Ca2+ channels in rat retinal arteriolar smooth muscle
McGahon, M.K.; Curtis, T.M.; McGeown, J.G.; Scholfield, Norman;
1. Vascular Biology, Queens University, Belfast, United Kingdom.
View other abstracts by:
Table 1. ID50s as µmol/l for the 3 modes of Ca2+ entry were read from the averaged curves using 4-14 microvessels. With CPA diltiazem** produced a 45±8% (S.E.M.) inhibition at low concentrations and the remaining Ca2+ influx requiring much higher concentrations
Where applicable, experiments conform with Society ethical requirements.