Leukocyte recruitment to sites of inflammation has been studied using a variety of experimental techniques including; intravital microscopy, static adhesion assays and flow-based adhesion assays using whole blood or isolated leukocytes. The development of fluorescent dyes that passively diffuse into cells and bind to intracellular compartments has enabled investigators to visualise interactions between leukocytes and endothelial cells (EC) using real-time imaging. However, there is little information on the detrimental effects that fluorescent dyes have on leukocyte behaviour in vivo or in vitro, which is an important factor in any study purporting to investigate their detailed adhesive behaviour.
In the present study we used two flow-based adhesion assays to investigate effects of high intensity illumination (HI light) on the migration behaviour of neutrophils in the presence or absence of five commonly used fluorochromes; Bisbenzimide (Hoechst 33342), Calcein-AM, CellTM Tracker Orange (CMTMR), Quinacrine and Rhodamine-6G.
The research was carried out according to local ethical guidelines in collaboration with South Birmingham’s Local Research Ethics Committee. Isolated neutrophils, with or without the fluorochrome labelling were either (1) perfused through P-Selectin (1 µg/ml) coated glass capillary tubes to establish a population of rolling cells and then activated by the bacterial peptide analogue formyl-methionyl-leucyl-phenylalanine (fMLP; 10-7 M), or (2) perfused through glass capillaries containing Human Umbilical Vein Endothelial Cells (HUVEC; donors gave informed consent) previously activated by the inflammatory cytokine tumour necrosis factor α (TNFα; 1 U/ml), so that the EC could support the adhesion and migration of flowing neutrophils. The research was carried out according to local ethical guidelines in collaboration with South Birmingham’s Local Research Ethics Committee.
All experiments were conducted on an Olympus Reflected Fluorescence System microscope and HI light was defined as light emitted from a USH10 100 W Mercury Burner with no barrier filters, and passed through the appropriate filters for Ultra-Violet (UV), blue and green light (excitation wavelengths; 330-385nm, 460-490 nm and 510-550 nm respectively) using a LCPlanFL 20 X 0.4 Ph1, CAP-P1.1±0.5 objective lense.
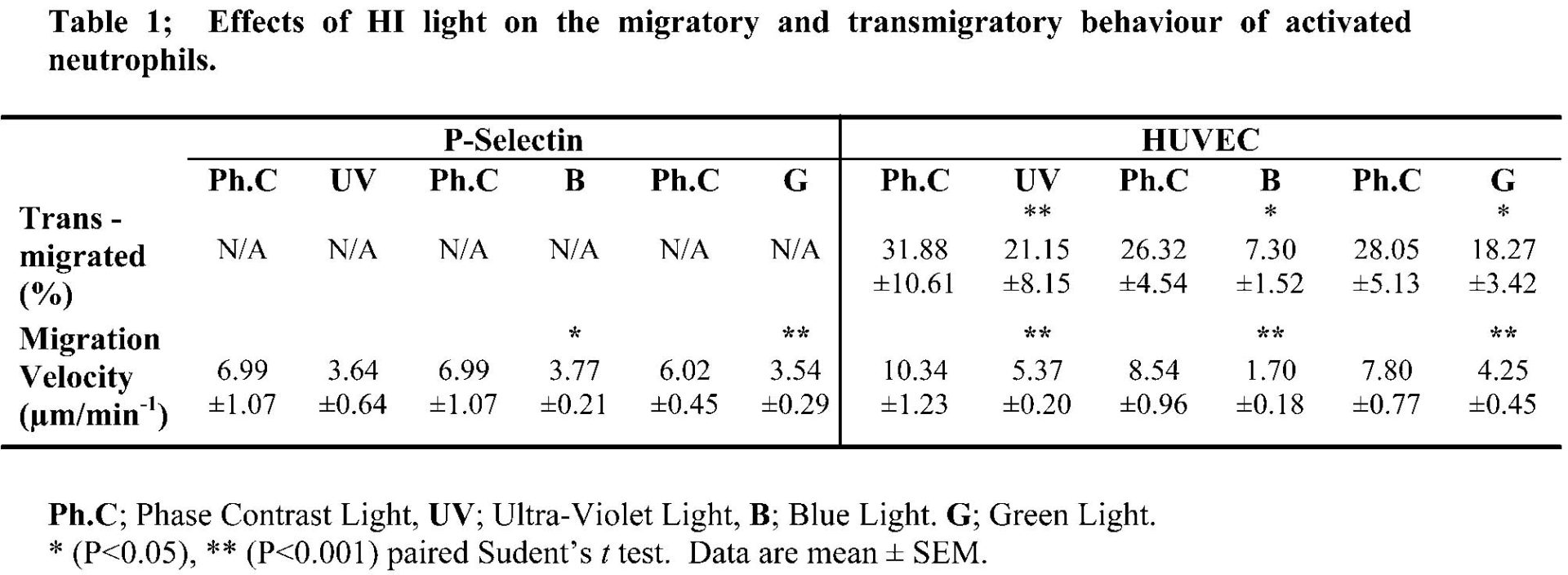
In the absence of HI light fluorochromes did not affect neutrophil behaviour. However, HI light alone caused a 50 % decrease in neutrophil migration velocities in both assays and caused a significant decrease in the number of neutrophils transmigrating through the EC monolayer. In the P-Selectin model, Calcein-AM, Cell Tracker Orange and Rhodamine-6G (0.25-4 µg/ml) in combination with HI light caused a decrease in migration velocities synergistic to the effects of HI light alone (P < 0.01). In the HUVEC model, the combination of HI light and Bisbenzimide, Calcein-AM and Cell Tracker Orange (1-4 µg/ml) caused a further decrease in the number of transmigrated neutrophils (P < 0.05), compared to Quinacrine which caused an increase (P < 0.05). Rhodamine-6G caused a decrease in migration velocity synergistic to that of HI light alone (P < 0.05).
It is therefore important that investigators examine the full effects that HI light and fluorochromes have on cell behaviour before employing them in experimental systems.
This work was supported by the BBSRC and AstraZeneca (CASE studentship 02/A4/C/08602).