Skeletal muscle mass progressively decreases with advancing age, increasing the risk of developing Sarcopenia, characterised by low appendicular muscle mass (Dufresne., 2016; Ageing and health., 2022). This currently poses a significant cost to global healthcare systems. Low skeletal muscle mass is more likely to cause a loss of independence and, ultimately, a reduced longevity. Osteoprotegerin (OPG), a decoy receptor for RANK/RANKL is known to prevent bone resorption within the bone remodelling cycle, yet it has been suggested to exhibit protective effects on dystrophic skeletal muscle in animal models (Dufresne et al., 2015).
To further understand the potential of OPG as a therapeutic target to combat age related muscle loss, a better mechanistic insight is required. Therefore, we aimed to identify if OPG exhibits protective effects in C2C12 myoblasts in the presence of TNFα, used to represent the inflammatory response associated with ageing, having previously shown deleterious properties in C2C12 cells. To achieve this aim we had 3 objectives 1; Ascertain concentrations of both TNFα and OPG that will exhibit observable effects on C2C12 myogenic differentiation, 2; develop a protocol to grow and treat differentiating C2C12 myoblasts with OPG and TNFα and, 3; treat C2C12 myotubes with TNFα and OPG, simultaneously, in order to examine any effect OPG may exhibit on differentiation parameters.
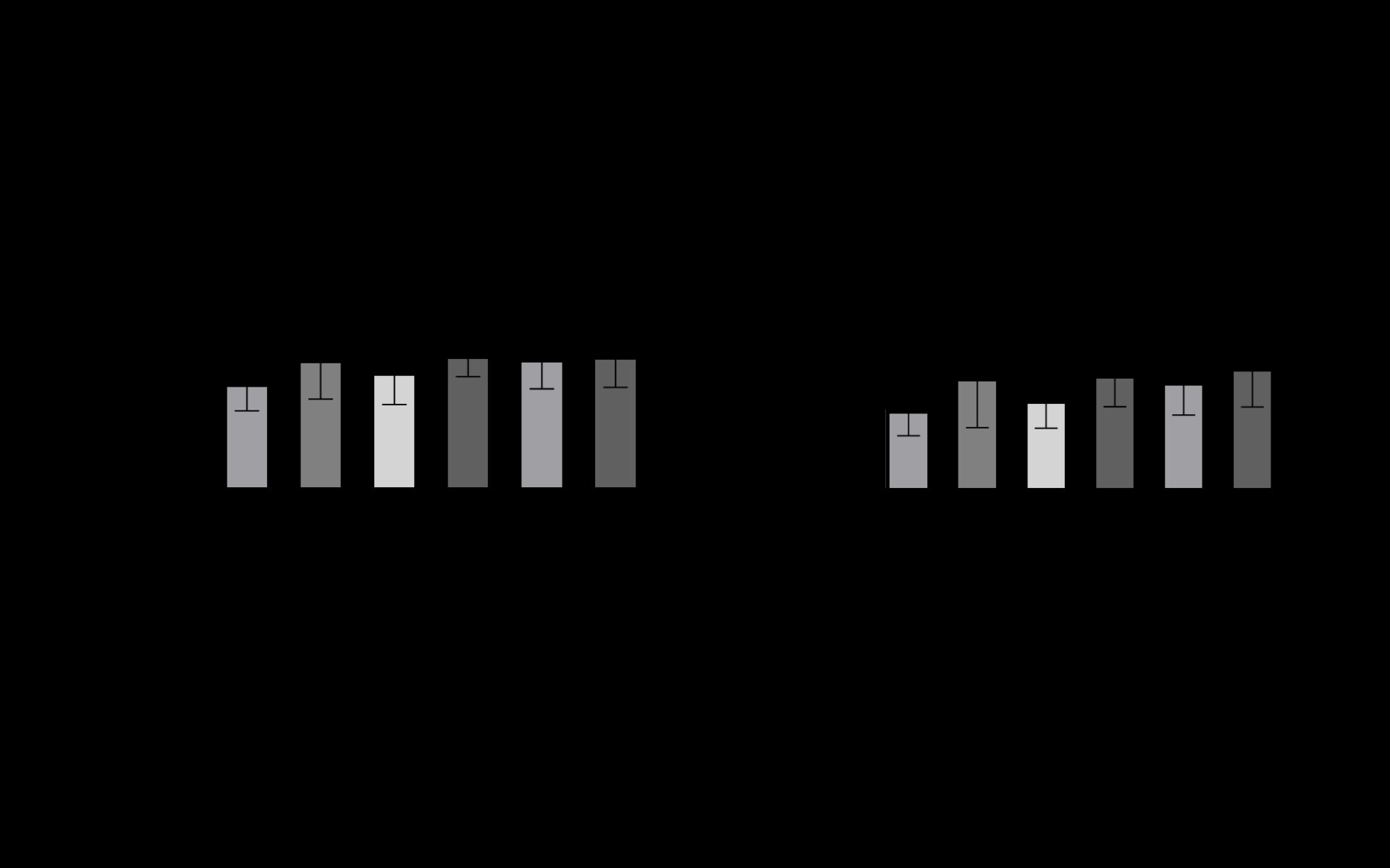
C2C12 myoblasts were seeded at a density of 5000 cells/cm2 in growth media (GM) and grown until confluent. A titration of OPG and TNFα was conducted, concluding with 30ng/ml-1 TNFα and 20ng/ml-1 OPG as the most effective treatment doses for differentiation. Cells were then seeded in 24 well plates and grown to confluence in GM (n=4) after which GM was replaced with Differentiation Media (DM). Experimental treatments were then added at 0 and 24 hours of incubation to observe OPGs effects on the formation of myotubes with TNFα present. Mean myotube diameter and mean number of myonuclei per myotube were measured as differentiation measurement parameters. Mixed effects analysis of variance and One-way ANOVA tests were used and significance accepted at p<0.05.
Between controls (DM) and groups treated with TNFα and OPG simultaneously, significant differences in mean myotube diameter and mean number of myonuclei/myotube were observed (p<0.05). In all conditions containing both TNFα and OPG, mean myotube diameter was non-significantly increased when compared to conditions containing only OPG or TNFα (p>0.05). Significant increases in mean number of myonuclei per myotube between groups treated with TNFα or OPG at 0h, and groups treated with TNFα or OPG at 0h followed by OPG or TNFα at 24h (p<0.05) were also observed (Figure 1). The data herein provides a sound, viable method for investigating the effects TNFα and OPG exhibit on differentiating C2C12 myotubes. In addition, OPG demonstrates protective effects on C2C12 differentiation in a model replicative of the ageing skeletal muscle. It is clear OPG has the potential to interact with bone and muscle, however, further insight is required to understand its mechanistic actions to confirm OPG as a therapeutic target to help overcome the musculoskeletal declines throughout the ageing process.