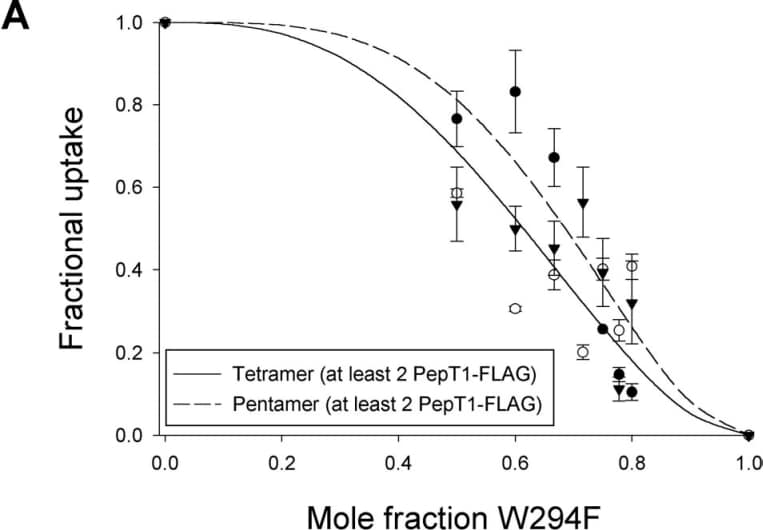
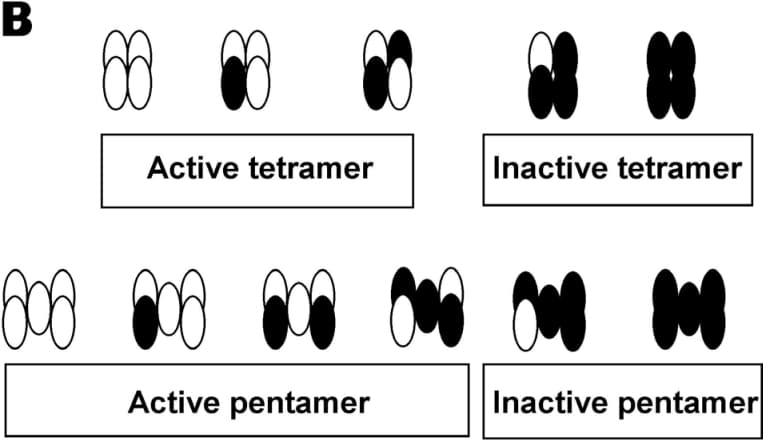
The proton-coupled transporter PepT1 mediates the uptake of di-, tri-peptides and peptidomimetics in the intestine and kidney (reviewed by Meredith & Boyd 2000). Here we report the results of studies to test whether PepT1 acts as a functional monomer or multimer, using a combination of luminometry and transport assays in Xenopus laevis oocytes.Uptake studies were performed as previously reported (Meredith et al. 2000). The epitope FLAG (YDDDDDK) was inserted by PCR (at amino acid position 108, in the extracellular loop between TM3 and TM4, Covitz et al. 1998) to enable measurement of its expression level at the oocyte membrane by luminometry (Konstas et al. 2001). PepT1-FLAG showed normal expression and function compared to the non-tagged wild type transporter. In contrast a W294F-PepT1 mutant was expressed on the membrane but did not transport the dipeptide D-Phe-L-Gln.Whilst a constant amount of PepT1-FLAG cRNA (13.5 ng) was co-injected with an increasing amount W294F mutant cRNA (up to a 1:4 ratio), the amount of D-Phe-L-Gln transport was reduced, yet the expression levels of PepT1-FLAG remained constant. This implies that PepT1 functions as a multimer as co-expression of the non-functional W294F affects uptake by PepT1-FLAG. Data from a series of co-injection experiments were fitted with the Hill equation, and the stoichiometry determined to be 4.2±1.8, suggesting that PepT1 functional unit may be composed of 3 (min) to 7 (max) copies of the protein. Data were normalized and plotted as the fractional uptake versus the mole fraction of the mutant (Figure A) and two curves were found to fit the data equally well. These two curves represent the tetramer and the pentamer model, where two copies of PepT1-FLAG appear to be the minimal requirement for function (Figure B).
University of Glasgow (2004) J Physiol 557P, C56
Communications: The rabbit proton-coupled peptide transporter PepT1 functions as a multimer when expressed in Xenopus oocytes
K.E. Panitsas, R. Boyd and D. Meredith
Human Anatomy & Genetics, University of Oxford, Oxford, UK
View other abstracts by:
Figure A:Normalized data plotted as the fractional uptake of 3H-D-Phe-L-Gln (0.42µM) versus the mole fraction (W294F/PepT1-FLAG+W294F) of the mutant. Data are mean ± SEM, n=5 oocytes. Lines represent theoretical fit for tetramer and pentamer.
Figure B:Possible ways that the PepT1-FLAG (open circles) and the W294F (filled circles) can be arranged in cases of tetramer and pentamer.Other 12 transmembrane domain transporters have recently shown to be multimers, including the human dopamine transporter (Hastrup et al. 2001). This study shows that PepT1 functions as a multimer, which will be highly relevant for structure-function modeling of the protein.
Where applicable, experiments conform with Society ethical requirements.