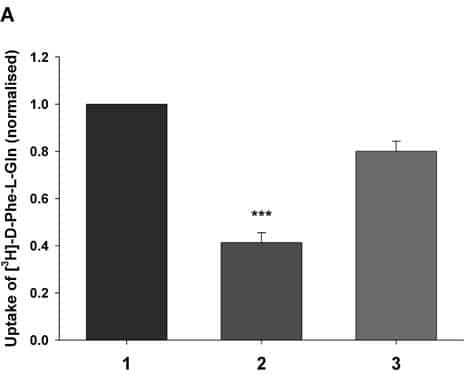
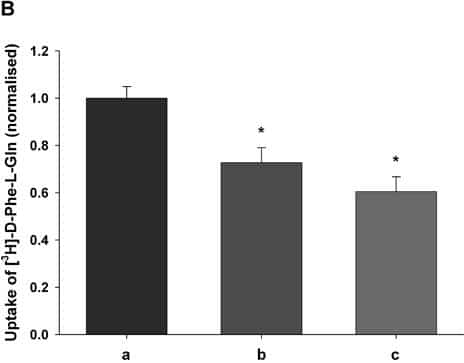
PepT1 mediates the intestinal absorption and renal re-absorption of di- and tri-peptides and a great number of therapeutically active compounds (Daniel & Kottra, 2004). Recently we have shown that PepT1 forms a multimer when expressed in Xenopus oocytes (Panitsas et al. 2006) using coinjection of an epitope-tagged wild-type protein (PepT1-FLAG) and a non-functional mutant W294F-FLAG. However, we could not exclude the possibility that other proteins may also be involved. To further investigate this, we have used rat intestinal RNA depleted of PepT1 message to see if there are other protein(s) expressed that could be involved. PepT1-FLAG was expressed in Xenopus oocytes as previously described (Panitsas et al. 2006), either alone or by coinjection as described in the figure legend. Rat intestinal RNA was prehybridised with antisense oligonucleotides to rat PepT1, NHE3 and NHERF2 using the method described by Fei et al. (1994). Both membrane expression and the transport were determined by luminometry and uptake of 0.4μM [3H]-D-Phe-L-Gln, respectively, using techniques previously described (Panitsas et al. 2006). Data are means ± SEM of n oocyte preparations. Figure 1A shows the ability of co-injected PepT1-depleted intestinal RNA to reverse the inhibition of W294F-PepT1 on PepT1-FLAG, suggesting that there is a protein normally expressed in intestine that interacts with PepT1. Figure 1B shows that prehybridising the intestinal RNA with antisense oligonucleotides to the candidate proteins of the Na+—H+ exchange system, NHE3 and NHERF2, abolishes the reversal of the inhibition seen in Fig. 1A, suggesting that these proteins are involved. Western blotting of immunopurified PepT1-FLAG (data not shown, n=3) gave bands which were consistent with a range of molecular complexes of PepT1 ranging from monomers to tetramers. In addition there were bands visible that were not multiples of the expected PepT1 monomer size of 76kDa (at 88, 156 and 262KDa), which could represent heteromultimers of PepT1 with other protein species. Further studies such as co-immunoprecipitation will be required to elucidate whether PepT1 and NHE3 and/or NHERF2 form a physical association or a functional one.
University of Manchester (2006) Proc Physiol Soc 2, PC34
Poster Communications: The rabbit proton-peptide cotransporter, PepT1, interacts with the sodium-hydrogen exchanger NHE3 and its regulatory factor NHERF2 when expressed in Xenopus oocytes
CAR Boyd1, Konstantinos-Emmanuil Panitsas1, David Meredith1
1. Physiology, Anatomy & Genetics, University of Oxford, Oxford, United Kingdom.
View other abstracts by:
Figure 1A. Uptake of D-Phe-L-Gln normalised to transporter surface expression in Xenopus oocytes expressing (1) PepT1-FLAG (2) PepT1-FLAG and W294F-PepT1 or (3) PepT1-FLAG and W294F-PepT1 when coinjected with PepT1-depleted intestinal RNA (*** p<0.001 Student’s t test n=13).
Figure 1B. Uptake of D-Phe-L-Gln normalised to transporter surface expression after coinjection of (a) PepT1-FLAG W294F-PepT1 and PepT1-depleted intestinal RNA additionally prehybridised with (b) NHE3 or (c) NHERF2 antisense oligonucleotides (* p<0.05 Student’s t test n≥3).
Where applicable, experiments conform with Society ethical requirements.