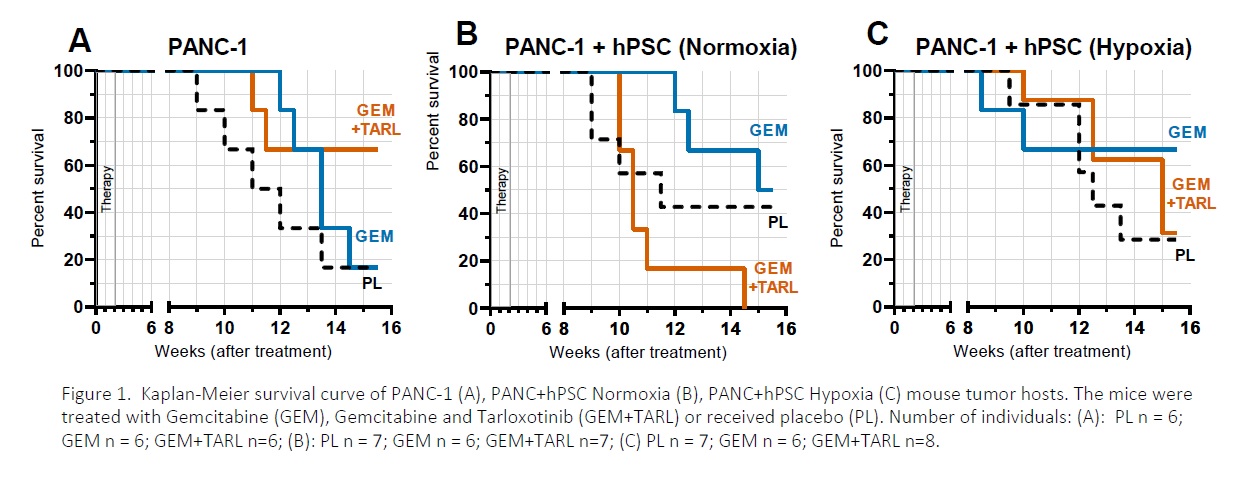
Pancreatic cancer affects as much as 400 000 people worldwide. This cancer is estimated to become the third most common type of cancer. Despite the development of the novel anti-cancer therapeutic strategies, survival rate of the patients did not increase significantly. Due to the high resistance of pancreatic cancer to chemotherapy and radiotherapy, or combination of these, pancreatic cancer remains one of the major unresolved health problems, and is characterized by one of the lowest of all cancers survival rates after diagnosis (1). In this work, we focus mainly on the role of hypoxia (poor oxygenation) in the tumor microenvironment (the stroma). By forming the physical barrier between cancer cell-normal cell, stromal component limits the effectiveness of anti-cancer treatment. Activated pancreatic stellate cells (PSCs) have been implicated into the hypoxia-mediated production of a dense fibrous stroma (the matrix), that in pancreatic cancer can constitute up to 80% of the tumor mass (2-4). In this study we used BALB/c nude mice as the hosts for the solid human pancreatic tumors. The mice were injected subcutaneously with the human pancreatic adenocarcinoma cells PANC-1 with or without the human pancreatic stellate cells (hPSC). The latter cells were cultured under normoxic (21% O2) or hypoxic (0.5% O2) conditions 24 h prior the injection. The tumors were treated with gemcitabine (GEM, 50 mg/kg, intraperitoneal injection) twice a week for two weeks, with or without Tarloxotinib, a hypoxia-activated pro-drug (GEM+TARL, 30 mg/kg, intraperitoneal injection); the control tumors received placebo (PL). Each experimental group consisted of 6-8 tumors. Tumor growth was monitored for 16 weeks (Figure 1): the mice were weighed and the tumor size was measured using calipers. After the experiment, the mice were humanely killed using CO2, the tumors and organs were excised, formalin-fixed and paraffin-embedded for immunohistological analysis. The animal experiments were approved by the local ethical committee, licence 41/2019. The quantitative results were expressed as means ± sem. The statistical significance threshold was set at 0.05. Analysis of the PANC-1 tumor host survival (Figure 1A) shows that the monotherapy with GEM (blue) is less effective than the combined therapy GEM+TARL (orange). In that contained the stromal component (Figure 1B and 1C) , monotherapy with GEM (blue) seems to be more effective than in the PANC-1 group (see Figure 1A). However, the combination therapy (orange) in the normoxic group (Figure 1B) was ineffective and mouse hosts of these tumors did not survive to the experiment end. Also, the combination therapy (orange) was less effective than the monotherapy (blue) in the group wherein PSCs were pre-treated with low O2 (Figure 1C). Immunohistological analysis of the tumor tissue revealed the increased level of the αSMA-positive cells in the tumors collected form the hosts of the best survival (Figure 1, blue). Taken together, our study explores the role of hypoxic stress in the pancreatic tumor growth and its response to the therapy.
Physiology 2021 (2021) Proc Physiol Soc 48, PC100
Poster Communications: The role of hypoxia in mouse pancreatic carcinogenesis – Effect of limited oxygen availability on the effectiveness of anti-cancer therapy
Daria Krzysztofik1, Agnieszka Kusiak2, Kinga Stopa1, Paweł Ferdek2, Ewa Werner1, Karolina Hajduk1, Jan Morys1, 2, Sylwester Mosiołek1, 2, Filip Łoziński1, 2, Monika Jakubowska1
1 Małopolska Centre of Biotechnology, Jagiellonian University , Kraków, Poland 2 Faculty of Biochemistry, Biophysics and Biotechnology, Jagiellonian University , Kraków, Poland
View other abstracts by:
Where applicable, experiments conform with Society ethical requirements.