Sarcopenic obesity, characterised by excess adiposity and diminished skeletal muscle mass and function, is associated with frailty and inflammation. Previous work from our group1 implies a role for dysregulated crosstalk between adipose tissue (AT) and skeletal muscle (SkM) in driving sarcopenic obesity, and increasing evidence demonstrates that extracellular vesicles (EVs) facilitate intercellular communication2. We therefore aimed to characterise AT-derived EV profiles across lean and obese subjects, map these to parameters of inflammation and adiposity, explore their effects on SkM, and identify mechanisms of action.
Adipose conditioned media (ACM) was generated from human ex vivo tissue explants collected during joint replacement surgery, and EVs were extracted from conditioned media by overnight ultracentrifugation at 100,000 x g. EV profiles were analysed using ExoView, nanoparticle tracking analysis, nano-flow cytometry, and small RNA sequencing. Human primary SkM myoblasts were isolated from SkM tissue collected peri-operatively during joint replacement surgery. Myoblasts were cultured to confluence before differentiation for 8 days to achieve elongated, multinucleated myotubes. Myotubes (n=3 from donors aged >60, n=3 from donors aged <60) were treated for 24 hr under the following conditions: vehicle PBS control, TNFa positive control, lean ACM, obese ACM, obese EV, and obese EV-depleted ACM. Myotube thickness and nuclear fusion were assessed as functional readouts, and RNA was extracted from treated myotubes to assess modulation of gene expression.
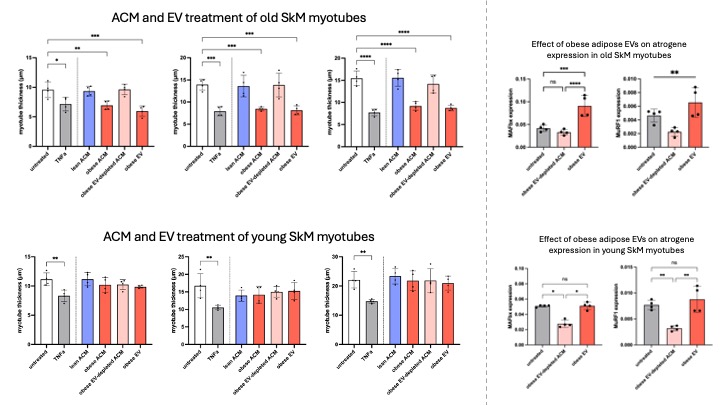
ExoView indicated classical tetraspanin EV marker expression within the population (n=15). Obese AT released fewer EVs than lean AT, with consistently higher EV yield from visceral AT compared to subcutaneous AT (n=5), supported by EV-associated protein concentration trends. Small RNA sequencing of AT-derived EVs reveals 7 differentially expressed microRNAs between lean and obese donors (n=15). In myotubes, TNFa, obese ACM, and obese AT-derived EVs (from a pooled source, n=5) all significantly reduced thickness in cells from old donors, but not young donors, and increased expression of atrophic and inflammatory genes compared to untreated vehicle control, lean ACM, and obese EV-depleted ACM treatments. This suggests that the EV fraction of obese ACM drives modulation of catabolic and anabolic pathways mediating SkM atrophy. Interestingly, concurrent treatment of old myotubes (n=1) with obese AT-derived EVs and an antagomir for key microRNAs upregulated in obese AT-derived EV partially yet significantly ablates the EV-induced elevation of the ubiquitin E3 ligase MAFbx.
The EV fraction of obese ACM appears critical in driving atrophic phenotypes and genotypes in sarcopenic human primary myotubes, with key microRNAs potentially mediating this effect. Interrogating these molecular pathways further in the contexts of adiposity, exercise-like stimulation, and age-related muscle loss will advance understanding of mechanistic drivers of sarcopenic obesity.