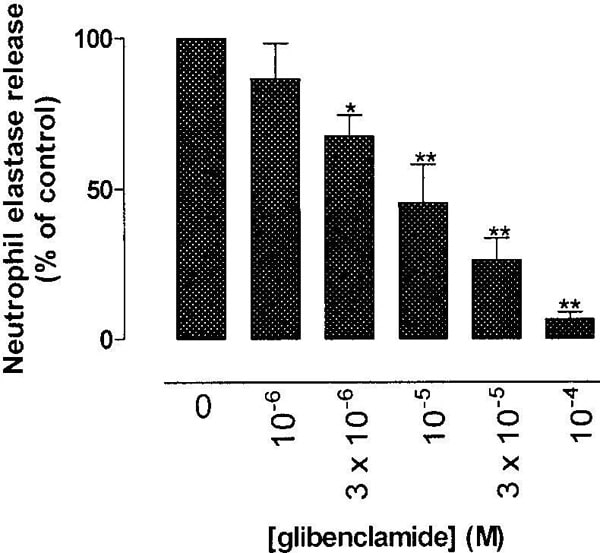
Inappropriate neutrophil activation and subsequent proteolytic enzyme release has been implicated in the pathogenesis of chronic inflammatory conditions such as chronic obstructive pulmonary disease (Brown et al, 2003). Neutrophil activation is dependent upon an increase in the intracellular concentration of Ca2+, mediated by store operated Ca2+ entry (SOCE; Itagaki et al, 2002). Human neutrophils express a range of K+ ion channels (Gallin, 1991), inhibition of which would be expected to produce membrane depolarisation, inhibit SOCE and consequently neutrophil activity. We have tested this hypothesis by studying the effects of drugs known to block K+ channels on neutrophil elastase (NE) release from fMLP-activated human neutrophils using methods described previously (Brown et al, 2003). Neutrophils were purified from peripheral venous blood collected from healthy human volunteers and resuspended ( 2.5 x 106 cellsml-1) in Hanks’ balanced salt solution. 150ml of this cell suspension was placed in each of a number of microcentrifuge tubes containing cytochalisin B (final concentration; 5 mgml-1) along with different concentrations of the drug under study (or vehicle). Cells were primed by the addition of TNF-α (10ngml-1; 30 min at 37 °C) before being activated by fMLP (10-7M; 45 min). The samples were centrifuged and 25 ml of the supernatant placed into the respective wells of a 96-well microplate. 150 ml of TRIS buffer was added to each well along with 20 ml of NE substrate N-ethoxysuccinyl-ala-ala-pro-val p-nitroanilide. After 30 min at room temperature the amount of the coloured elastase product, proportional to the amount of NE present, was quantified by measuring absorbance at 405 nm in a microplate reader. Data were normalized and expressed as percentage inhibition of the NE release obtained from neutrophils in the absence of drug after correction for basal NE release. Data was analyzed using ANOVA and Dunnet’s post hoc test with P<0.05 considered significant. All experiments were repeated three times in duplicate. NE release was inhibited in a concentration dependent manner by both the calcium entry blocker SKF96365 (1-100 μM; IC50 16 μM; maximum inhibition 96.0±0.5%) and glibenclamide (1-100 μM; IC50 7 μM; maximum inhibition 93.6±2.3%. Fig 1). Another sulphonylurea, glipizide was also effective but only at the highest concentration tested (32.5±5.1% inhibition at 100μM). The K+ channel blockers tetraethylammonium (TEA; 0.1–10 mM), 4 aminopyridine (4-AP; 1 μM–1 mM) and verapamil (1–100 μM) had no significant effect on NE release. These results suggest ATP-dependent potassium channels play a role in regulating neutrophil activity and that blockers of these channels could offer a novel means of inhibiting neutrophil function.
Life Sciences 2007 (2007) Proc Life Sciences, PC110
Poster Communications: The sulphonylurea glibenclamide inhibits fMLP-activated elastase release from human neutrophils
H. Wileman1, R. A. Brown1, I. McFadzean1
1. Sackler Institute of Pulmonary Pharmacology, King's College London, London, United Kingdom.
View other abstracts by:
Figure 1: Effects of glibenclamide on NE release from human neutrophils. *P<0.05; **P 0.01 compared to control.
Where applicable, experiments conform with Society ethical requirements.