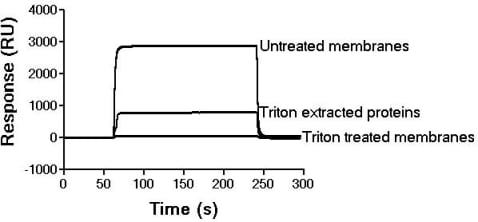
The thyroid hormones T3 and T4 translocate the plasma membrane through multifunctional transporter proteins (Hennemann et al, 2001). In liver cells, the hormones may bind Triton X-100 soluble receptor proteins in the plasma membrane that channel T3/T4to their transporters (Kemp & Taylor, 1997). A novel approach to studying this type of interaction is surface plasmon resonance (SPR) in which one member of a binding pair is immobilised to a sensor surface and the second member is passed over the immobilised partner. Binding is detected in real time as a change in refractive index at the sensor surface and is proportional to the change in mass due to binding.Sinusoidal membranes purified from livers of humanely-killed rats (Kemp & Taylor, 1997) were immobilised on an L1 sensor chip placed in a Biacore 3000 SPR system (Biacore AB, Uppsala, Sweden). Micromolar concentrations of T3,T4 and rT3 in phosphate-buffered saline were injected over the immobilised membranes. SPR sensorgrams were double referenced with subtraction of non-specific binding to an unmodified sensor surface. Concentration dependent binding of T3 and T4 to the membranes was observed and analysed by a 1:1 Langmuir adsorption model using BioEvaluation software. For T3; ka = 1.51 (±0.01) x 104 Ms-1, kd = 0.029 (±0.004) s-1, KD = 1.89 µM. For T4; ka = 1.36 (± 0.16) x 104 Ms-1, kd = 0.171 (±0.015) s-1, KD = 12.6 µM. Here ka and kd represent the on/off rate constants of the interaction while KD is the equilibrium dissociation constant. rTdid not interact with liver membranes.T3 – receptor interactions were also studied by injecting liver membranes over T3 immobilised to a CM5 sensor surface (0.8 pmol.mm-2); 15 µg membrane protein produced a maximum specific binding response (Figure 1). Membranes briefly treated with 0.5% Triton X-100 to solubilise T3 receptors without loss of membrane vesicular integrity (Kemp & Taylor, 1997) showed a 98% reduction in binding. Crude Triton-extractable membrane protein displayed binding activity towards T3 bound to the CM5 chip (see Figure 1). These results provide valuable kinetic information on real-time binding interactions of T3 and T4 with components of liver sinusoidal membrane. This work has demonstrated that SPR is of value in the identification and kinetic characterisation of physiologically important receptor – ligand binding interactions.
University of Glasgow (2004) J Physiol 557P, C53
Communications: Thyroid hormone binding interactions with rat liver membranes investigated using Surface Plasmon Resonance.
A. McBride and P.M. Taylor
Division of Molecular Physiology, University of Dundee, Dundee, UK
View other abstracts by:
Figure 1. SPR sensorgram of interactions between T3 immobilisedto a CM5 surface and superfused liver sinusoidal membrane material.RU:- SPR Response Unit
Where applicable, experiments conform with Society ethical requirements.