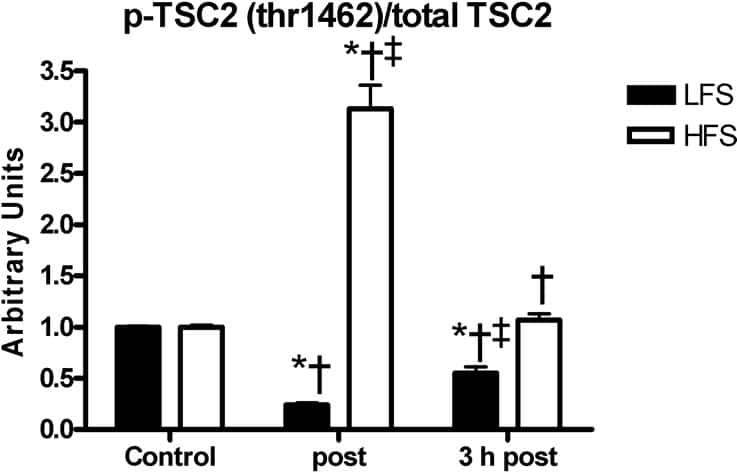
We have previously reported a switching behaviour to either AMP kinase (AMPK) or protein kinase B (PKB) signalling in response to endurance and resistance training-like muscle stimulation. Others have shown that tuberin (TSC2) could be phosphorylated by AMPK (Inoki et al., 2003) and PKB (Manning et al., 2002). The aim was to investigate whether the observed AMPK-PKB effects were reflected by variations in TSC2 Thr1462 phosphorylation in response to endurance and resistance training-like stimulation. Male wistar rats were humanely killed and their rat extensor digitorum longus (EDL) or soleus muscles were incubated in oxygenated Krebs-Henseleit buffer and electrically stimulated either continuously at 10 Hz for 3 h (endurance training-like low frequency stimulation; LFS) or for 10 sets of 6 repetitions of 3 s-100 Hz bursts (resistance training-like high frequency stimulation; HFS). Muscles were frozen, protein extracted and Western blotting was carried out using primary antibodies against phospho-TSC2 (Thr1462) and total TSC2. TSC2 phosphorylation at Thr1462 (normalized to total TSC2) increased significantly to 3.13±0.23 directly after HFS but was not significantly different 3 h thereafter (Figure 1). In contrast, TSC2 Thr1462 phosphorylation decreased significantly to 0.24±0.02 directly and 0.55±0.06 3 h after LFS. Total TSC2 decreased significantly to 0.82±0.02 directly after HFS and increased to 1.27±0.02 after LFS but was not significantly different 3 h after stimulation. TSC2 Thr1462 phosphorylation is both sensitive to LFS and HFS. The previously reported PKB activation by HFS can explain the acutely increased TSC2 Thr1462 phosphorylation. The LFS effect on TSC2 Thr1462 phosphorylation is not PKB-induced because PKB phosphorylation was not affected by LFS. However, AMPK which is activated by LFS, phosphorylates TSC2 at Thr1227 and Ser1345 (Inoki et al., 2003). The AMPK-phosphorylated sites are close to the Thr1462 site and their phosphorylation by AMPK could potentially inhibit Thr1462 phosphorylation. The total TSC2 data support the hypothesis that Thr1462 phosphorylation controls TSC2 degradation (Inoki et al., 2002). To conclude, the inhibitory effect of LFS and activating effect of HFS on downstream regulators of protein synthesis is likely to be mediated via TSC2 Thr1462 phosphorylation and TSC2 content. TSC2 is thus the downstream executer of a signalling switch between AMPK and PKB-dependent signalling in response to LFS and HFS, respectively.
University of Nottingham (2004) J Physiol 558P, C2
Communications: TSC2 phosphorylation at Thr1462 increases in response to resistance and decreases in response to endurance training-like stimulation in rat skeletal muscle
Atherton,Philip J; Singh,Jaipaul ; Wackerhage,Henning ;
1. University of Dundee, Dundee, United Kingdom. 2. University of Central Lancashire, Preston, United Kingdom.
View other abstracts by:
Figure 1 TSC2 phosphorylation at Thr1462/TSC2. (mean ± SEM; n=8) (p<0.05).*Significantly different from control; †significant difference between LFS and HFS; ‡Significantly higher in EDL.
Where applicable, experiments conform with Society ethical requirements.