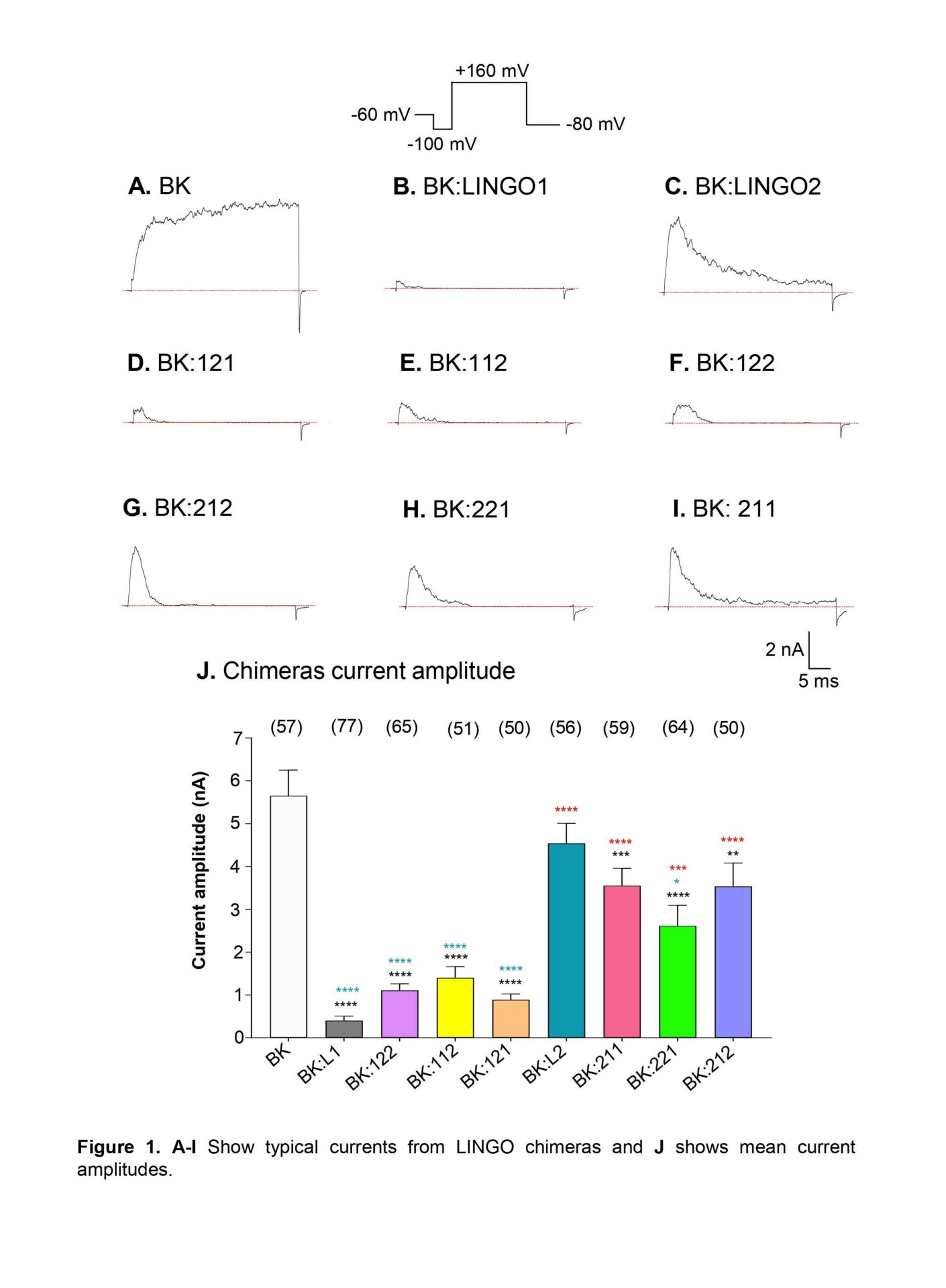
BK channels are ubiquitously expressed and alterations in their expression was associated with motor disorders1-3. Their biophysical properties are modulated by splice variants as well as auxiliary β, γ, and LINGO subunits4. LINGO proteins are Leucine Rich Repeat proteins with an extracellular (ED), transmembrane (TM) and cytosolic tail (TD) domain3-5. BK co-expression with LINGO1-2 resulted in inactivating BK currents and shifted their half maximal activation voltage (V1/2ACT) negatively (~-50mV and ~-30mV, respectively)3,5. However, unlike LINGO2, BK co-expression with LINGO1 reduced their plasmalemmal expression3,5. We exploited the differences between LINGO1&2 via using six chimeras to identify the domains linked with the reduction in BK expression and the negative shift in V1/2ACT. Chimeras were named based on the origin of their ED, TM or TD.
The inside-out configuration of the patch clamp technique was used on HEK cells co-transfected with BK cDNA with either LINGO1 or the chimeras (300:300 ng) or with BK and LINGO2 cDNA (100:500 ng) using equimolar 140 mM K+ solutions, at 37°C, with 100 nM-10 µM Ca2+ applied to the patch cytosolic surface. Currents were evoked by a step to +160 mV for 40 ms, with 5 MΩ pipettes in 100 nM Ca2+. Steps from -100 mV to +200 mV in 20 mV increments were applied and the currents utilised to generate GV curves. Data is presented as mean ± SEM and statistical significance tested using one-way ANOVA.
Inactivation was observed with all chimeras co-expressed with BK (Figure 1) and the mean current amplitude for BK was 5650±600 pA (n=57). Co-expression with LINGO1 caused a ~93% reduction in current amplitude (401±105 pA, n=77, p<0.0001). A significant reduction in current amplitude was noted for BK:121 (888±136 pA, n=50), BK:112 (1398±260 pA, n=51) and BK:122 (1107±150 pA, n=65), when compared to BK (p<0.0001). In contrast, no significant difference was observed for BK:LINGO2 (4538±471 pA, n=56) compared to BK as shown previously5. Currents from BK:221 patches had smaller current amplitudes (2614±479 pA, p<0.05) than BK:LINGO2.
The V1/2ACT of all chimeras with a LINGO1 TD (BK:211, 75±2 mV, n=12; BK:221, 71±2 mV, n=6; BK:121, 69±3 mV, n=9) were similar to BK:LINGO1 in 1 µM Ca2+ (76±3 mV, n=11, p≥0.6938). The V1/2ACT for BK:LINGO2, 51±2 mV (n=6) and all chimeras with a LINGO2 TD in 1 µM Ca2+ were all similar (BK:122, 44±4 mV, n=7; BK:112, 40±4 mV, n=6; BK:212, 48±3 mV, n=8; p≥0.8249).
Deleting the last four C-terminus residues (BK:211DMKMI) abolished inactivation and the V1/2ACT was 90±3 mV in 100 nM Ca2+ (n=6). Similarly the V1/2ACT of BK:211DRKFNMKMI, was 113±2 mV in 100 nM Ca2+ (n=8), suggesting that the negative shift in activation was retained in the absence of the last eight residues.
These data suggest that the extracellular domain of LINGO1 is responsible for the reduction in plasmalemmal BK expression. Although the TD of LINGO appears responsible for setting V1/2ACT in 1 µM Ca2+, the last eight residues of this region were not necessary for this.