Previously (Yeung & Robertson, 2003) we showed that delayed rectifier-type Kv3 channels were effectively blocked by the peptidic toxin blood depressing substance (BDS). Based on the data of Swartz & MacKinnon (1997), who demonstrated that particular residues on the Kv2.1 channel, when mutated to alanine, reduced sensitivity to block by the gating modifier hanatoxin (HaTx), we sought to identify the residues on Kv3.2 that may confer sensitivity to BDS.
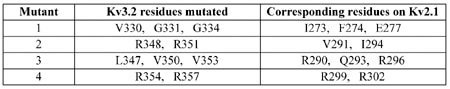
Four mutant Kv3.2 channels with multiple point mutations to alanine were generated by site-directed mutagenesis. The mutated sites on Kv3.2 (in the S3/S4 regions) were aligned to those on Kv2.1 that conferred HaTx sensitivity (Table 1).
Recordings were made from HEK293 cells using our previous methods (Yeung & Robertson, 2003).
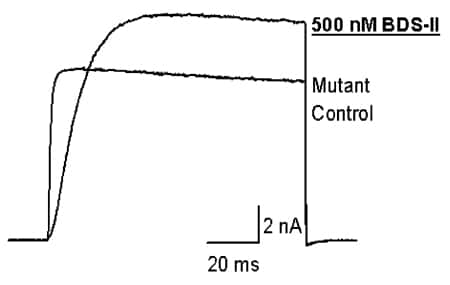
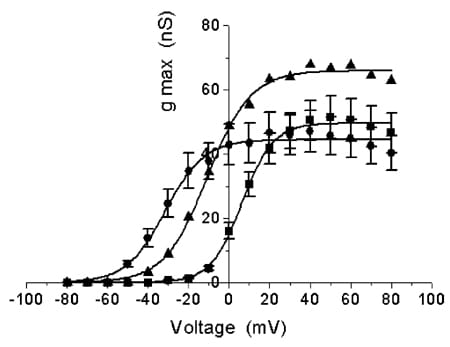
Of the four mutant Kv3.2 channels tested, mutant 3 was of particular interest. Under control conditions, mutant 3 showed a more hyperpolarised threshold (~-50 mV, shift of ~-30 mV) and a more rapid rate of current activation (2-fold change) (Fig. 1). BDS-II (500 nM), which blocks wild-type channels by 48.8 ± 4.3 % (n = 3) at +40 mV, now potentiated the current through mutant 3 channels (26.3 ± 5.3 % at +40 mV, n = 6 (means ± S.E.M.)).
This work is supported by the MRC and the Wellcome Trust.