Professor Khaled Machaca, Weil Cornell Medicine Qatar
The cellular and molecular world holds many clues to physiological and pathological conditions. Seeking to better understand our physiology and what make us sick, Professor Khaled Machaca (Weil Cornell Medicine Qatar) is fascinated with this intricate realm. Delving into cellular signals, Machaca and a team of researchers from Weill Cornell Medicine Qatar developed a new mouse model to help treat autoimmune and inflammatory diseases, recently published in The Journal of Physiology. Find out how exploiting nuances at the cellular and tissue levels can pave the way for the development of new drugs to treat chronic diseases, such as psoriasis, rheumatoid arthritis, and multiple sclerosis.
Studying disruption at the cellular level to understand human diseases
Our focus on studying cellular signalling stems from an interest to better understand the cellular and molecular mechanisms underlying normal physiological functions and how their disruption leads to disease or pathological states. In many cases understanding physiological homeostasis requires defining the signalling pathways involved that allow cells to respond and integrate various intracellular and environmental signals into the proper cellular response.
Defining signalling pathways and how they integrate goes a long way toward understanding normal physiology and by extension how it is altered in pathological conditions. The more detailed our understanding is, the more likely we will be able to intervene to treat disease states, and importantly to incorporate specificity in our approach to minimise side effects.
Biology reuses signalling molecules and pathways extensively, not only among different cells within the organism, but also between different species. Therefore, to develop a specific therapeutic approach one needs to understand the tissue specific nuances and exploit them.
Calcium ions and the vital role they play
Calcium is unique as a signalling molecule as it is an ion that cannot be metabolised. So, generating and removing a calcium signal requires the transport of calcium ions across cellular membranes that define specific intracellular compartments. Calcium ion signalling is widespread in practically every cell type and tissue. It is involved at some level in many physiological responses.
Understanding the mechanisms governing calcium ion signalling has broad implications for biology and physiology. As discussed above the molecules that mediate calcium signals are redundant and used in many cell types. This poses challenges in terms of understanding their cell-specific regulation and interactions, not only in the context of calcium signalling but also crosstalk with other signalling pathways.
Activation of immune cells
A specific calcium influx pathway at the cell membrane, known as store-operated calcium ion entry (SOCE), mediates entry of calcium ions into the cytoplasm of the cell from the extracellular space. SOCE is activated in response to calcium levels, within the endoplasmic reticulum (ER), which acts as the primary intracellular calcium store. The ER stores calcium to allow the cell to generate cytoplasmic calcium signals through intracellular release of calcium ions.
SOCE has been shown to be important for many physiological functions including activation of immune cells, secretion, and muscle development. This is supported by the clinical phenotype of patients with mutations in SOCE proteins, STIM1 or Orai1, who present with combined immunodeficiency due to the inability of their immune cells to activate, as well as muscle weakness.
Treating chronic disease
SOCE is important for immune cell activation and has thus been a therapeutic target for autoimmune and inflammatory diseases such as rheumatoid arthritis, pancreatitis, psoriasis, and multiple sclerosis. As most of these diseases tend to be chronic any potential therapeutic intervention would need to be for long periods of time.
If we are to target SOCE we need to understand the side effects of chronic SOCE inhibition. There are several animal models with tissue specific knockout of the proteins, STIM1 or Orai1. Unfortunately, these knockout models are not useful in defining potential toxicities associated with chronic SOCE inhibition because clinical inhibition with drugs would not lead to complete loss of SOCE. However, defining these toxicities are needed for new drug development to treat these autoimmune and inflammatory diseases.
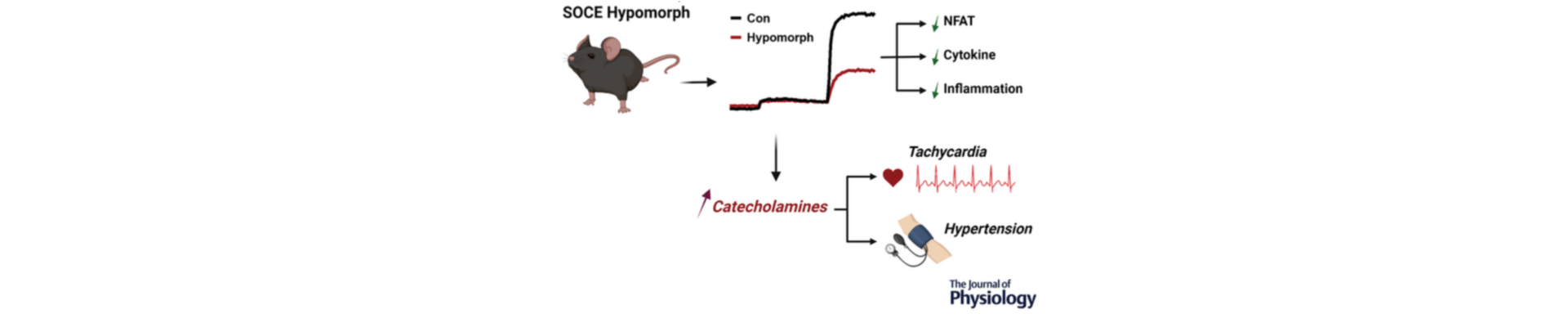
We developed a novel mouse model, that we refer to as the SOCE hypomorph. This mouse strain has decreased SOCE function (~65% inhibition) in the entire organism, so it provides a good model to understand the effects of long term SOCE inhibition.
A new mouse model for therapy interventions
Our studies show that long term SOCE inhibition is viable therapeutically, because in the hypomorph model SOCE was inhibited throughout the life of the animal. However, there was one drawback with the model as SOCE inhibition was associated with cardiovascular pathologies including hypertension and increased heart rate (tachycardia). The hypertension was likely due to the tachycardia.
To resolve the cardiovascular complications arising in our model, we investigated the cause of the tachycardia. We found that it is due to increased production of catecholamines (the hormones adrenaline and noradrenaline) in these animals One of the benefits of our mouse model is that we could create tissue specific hypomorph strains (a mouse line where the mutation is expressed only in one tissue rather than the whole animal). We developed a heart-specific SOCE hypomorph, that is a mouse strain where SOCE is inhibited only in the heart, while retaining normal SOCE levels in the other tissues. The heart-specific hypomorph did not show the cardiovascular pathologies, which suggests that the defect is not heart-specific but due to defects in other tissues.
What’s next for the SOCE hypomorph model?
We are excited that SOCE is a viable therapeutic target to treat autoimmune and chronic inflammatory diseases. We were also relieved to learn from the hypomorph model that chronic SOCE inhibition is not associated with increased susceptibility to diabetes, which is always a concern with these chronic treatments.
To explore the potential clinical application of our model, we would need to carry out further tests and work on translating these findings to clinical trials in humans in the context of specific chronic inflammatory diseases such as rheumatoid arthritis, pancreatitis, psoriasis, and multiple sclerosis. The SOCE hypomorph model can provide significant additional insights based on the specific disease to be targeted as one can generate tissue specific SOCE hypomorph strains to determine the effect of SOCE inhibition in a tissue specific fashion. This would allow for a more focused approach in terms of pharmacologically targeting the disease in question.
Read the full Research Article to find out more about the SOCE hypomorph mouse model and details of the results.