By Fulvia Draicchio, University of Roehampton, London, UK
Chronic kidney disease (CKD) is a long-term condition involving the gradual loss of kidney function over time.
CKD consists of five stages of kidney damage, from very mild in stage 1 to complete kidney failure in stage 5. People in stage 5 need to go on maintenance hemodialysis (MHD) treatment, which involves a dialysis machine to clean waste and extra fluids from patients’ blood.
Most MHD patients believe they cannot exercise. Disturbing data suggest that the levels of physical activity in this population is markedly decreased, to the extent that a MHD patient in his 30s is less physically active than a healthy sedentary person in his 70s (1, 2).
What affects the levels of physical activity in MHD people?
Under hemodialysis, these patients experience a unique form of protein and energy malnutrition, which causes a dramatic loss of 1 to 3 kilograms of muscle mass annually, reducing muscle strength and function, and ultimately leading to muscle wasting (3, 4).
As if that were not enough, muscle wasting involves various metabolic dysfunctions, including:
- metabolic acidosis, which occurs when too much acid is produced in the body because the kidneys are not working properly and cannot remove the acid in excess
- insulin resistance, a pathological condition where the body’s cells do not respond normally to the effect of insulin (a hormone produced by the pancreas responsible for the body’s use of glucose as energy), increasing the risk of developing other metabolic disorders such as type 2 diabetes and heart disease (4, 5)
- chronic inflammation
- reduced protein intake
- loss of nutrients and amino acids
Yet, the underlying mechanisms and key molecules responsible for this are unclear and there is currently no known treatment for muscle wasting in MHD patients.
In our recent study published in The Journal of Physiology, however, we found three potential candidates that may play a role in muscle wasting, and insulin and nutrient impairments in the kidney failure population: the integrin-liked kinase (ILK), PINCH and the focal adhesion kinase FAK.
These intracellular proteins are involved in the integrin signalling pathway, which connects the extracellular matrix (ECM) to the cytoskeleton (7).
The ECM is a highly-dynamic structure, which consists of a network of proteoglycans and proteins and it modulates biological processes including cell migration, differentiation, development and repair.
Moreover, the ECM is fundamental for cell-to-cell interaction as well as function and maintenance of all tissue, implying that ECM integrity is crucial for the proper function and stability of skeletal muscles (7, 8).
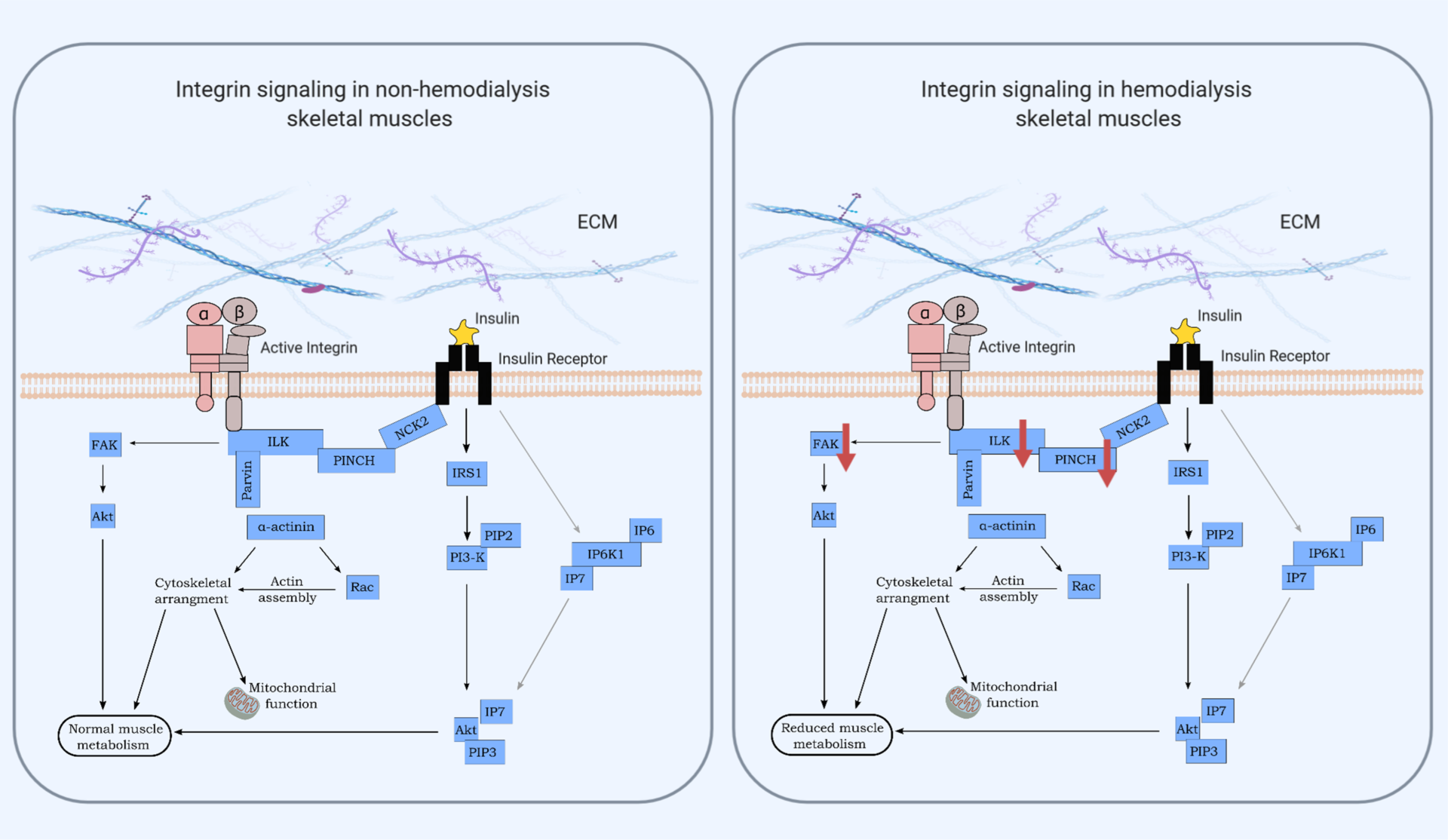
Integrins are major surface receptors of the extracellular matrix (ECM) in skeletal muscles and mediate interactions between the ECM and the cytoplasmic cytoskeleton (7, 9). Integrins are responsible for transducing signals across the membrane to the intracellular integrin-binding proteins, among which ILK, PINCH and FAK (Figure 5, 7, 10), which may be implicated in nutrient uptake and glucose metabolism in skeletal muscle.
Novel research suggests that a structurally stable link between the ECM and the cytoskeleton is required to maintain muscle proper functionality and encourage normal nutrient delivery and insulin sensitivity in skeletal muscles. On the other hand, any disruption of this link leads to muscle integrity and function impairment, followed by nutrient metabolism dysfunction and insulin resistance.
That said, we observed that skeletal muscles of insulin resistant MHD patients displayed markedly decreased ILK and PINCH levels as well as decreased FAK activity compared to non-hemodialysis individuals (Figure 1). The non-hemodialysis subjects, who were considered as the control group in this study, were not the portrait of health, though, since they were showing insulin resistance levels similar to the MHD group.
Interestingly, the decrease of these integrin-associated proteins was associated with a reduction in whole-protein metabolism (estimated using an infusion of labelled phenylalanine) 30 and 60 minutes after a meal in MHD patients compared to non-hemodialysis subjects, but both groups showed similar levels of insulin sensitivity and β-cell function.
Moreover, the tyrosine kinase Akt resulted unaltered between MHD and controls (Figure 1), suggesting that the integrin signalling may act independently from the traditional insulin-stimulated pathway involving PI3K/Akt, yet both pathways promote glucose uptake in skeletal muscles.
Taken together, our data suggest for the first time that key proteins involved in the integrin-cytoskeleton linkage are dysregulated in dialysis subject and may contribute to muscle wasting and nutrient impairment, therefore providing new molecular targets for future treatments for chronic kidney failure disease.
Please note that all views expressed on The Physiological Society’s blog reflect those of the author(s) and not of The Society.
References
- Shimoda T, Matsuzawa R, Yoneki K, et al. Changes in physical activity and risk of all-cause mortality in patients on maintence hemodialysis: a retrospective cohort study. BMC Nephrol. 2017;18(1):1-8. doi: 10.1186/s12882-017-0569-7
- Alp Ikizler T, Pupim LB, Brouillette JR, et al. Hemodialysis stimulates muscle and whole body protein loss and alters substrate oxidation. Am J Physiol – Endocrinol Metab. 2002;282(1 45-1):107-116. doi: 10.1152/ajpendo.2002.282.1.e107
- Robinson KA, Baker LA, Graham-Brown MPM, Watson EL. Skeletal muscle wasting in chronic kidney disease: the emerging role of microRNAs. Nephrol Dial Transplant. 2020;35(9):1469-1478. doi: 10.1093/ndt/gfz193
- van Vliet S, Skinner SK, Beals JW, et al. Dysregulated Handling of Dietary Protein and Muscle Protein Synthesis After Mixed-Meal Ingestion in Maintenance Hemodialysis Patients. Kidney Int Reports. 2018;3(6):1403-1415. doi: 10.1016/j.ekir.2018.08.001
- Ikizler TA, Himmelfarb J. Muscle wasting in kidney disease: Let’s get physical. J Am Soc Nephrol. 2006;17(8):2097-2098. doi: 10.1681/ASN.2006060629
- Draicchio F et al. Integrin‐associated ILK and PINCH1 protein content are reduced in skeletal muscle of maintenance hemodialysis patients. The Journal of Physology. 2020. doi: 10.1113/JP280441
- Williams AS, Kang L, Wasserman DH. The extracellular matrix and insulin resistance. Trends Endocrinol Metab. 2015;26(7):357-366. doi: 10.1016/j.tem.2015.05.006
- Mayer U. Integrins: Redundant or important players in skeletal muscle? J Biol Chem. 2003;278(17):14587-14590. doi: 10.1074/jbc.R200022200
- Postel R, Vakeel P, Topczewski J, Knöll R, Bakkers J. Zebrafish integrin-linked kinase is required in skeletal muscles for strengthening the integrin-ECM adhesion complex. Dev Biol. 2008;318(1):92-101. doi: 10.1016/j.ydbio.2008.03.024
- Kang L, Mokshagundam S, Reuter B, et al. Integrin-linked kinase in muscle is necessary for the development of insulin resistance in diet-induced obese mice. Diabetes. 2016;65(6):1590-1600. doi: 10.2337/db15-1434
