
Physiology News Magazine
40K: from ancient supernovae to bananas and beyond
Features
40K: from ancient supernovae to bananas and beyond
Features
Associate Professor Ronan Berg
Department of Clinical Physiology and Nuclear Medicine, Copenhagen University Hospital –Rigshospitalet, Copenhagen, Denmark
Department of Biomedical Sciences, Faculty of Health and Medical Sciences, University of Copenhagen, Denmark
“We are stardust / billion-year-old carbon.” Indeed, we are; as you sit down and read this article, I recommend that you put on Joni Mitchell’s track “Woodstock” from 1970 in which she sings these lines, with a voice that appears to vibrate through time and space into the endless universe. However, this is not an article about carbon, but about potassium, which like carbon was formed in generations of dying stars way back when. A trace amount of naturally occurring potassium throughout our Solar System, including in our bodies, is the unstable and thus radioactive isotope 40K. As it turns out, the presence of this isotope is a hint of the very birth of our Solar System.
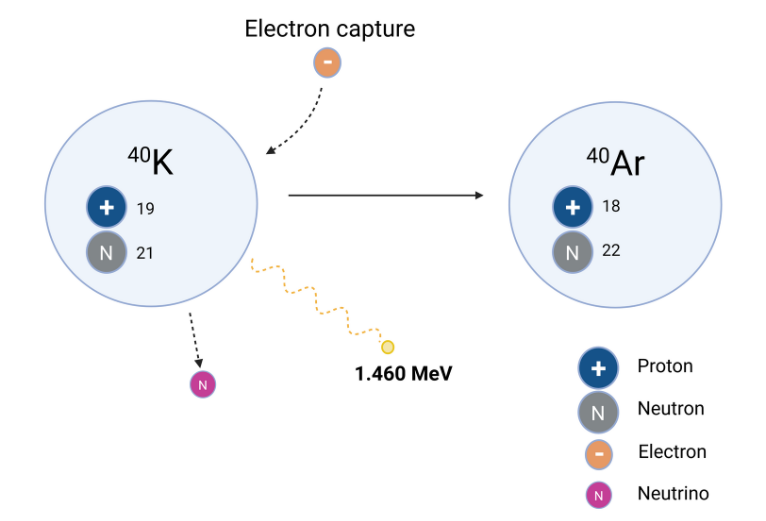
Due to concerns that working with radioactive materials would result in internal contamination, the first scintillation detectors were built into whole-body counters in the immediate post-World War II period and used for routine monitoring of nuclear workers. Somewhat surprisingly at the time, a high energy peak of ionising radiation at 1.460 MeV was persistently noted, indicating the omnipresence of the same radioactive isotope regardless of the worker’s exposure history to radioactive materials. It was subsequently clarified that this “background peak” was caused by the decay of 40K as it decays to 40Ar by electron capture (Fig.1).
Curiously, 40K has an astoundingly long – at least from a physiological perspective – half-life of nothing less than 1.251 billion years! So, at the current geological age (i.e. the Holocene), its proportion of total potassium is practically constant at 0.0117% (Bin Samat et al., 1997). In the context of physiology research, the detection of 40K by whole-body counting can be used to accurately determine whole-body potassium content without a need of any tracer injection or invasive sampling of any kind. It is a somewhat time consuming procedure (it takes approximately 45 minutes including patient preparation for a single whole-body 40K measurement at my own department), which largely hampers its use in clinical routine, and it is thus primarily used in research studies of electrolyte physiology and fluid homeostasis, in which it is particularly useful, because serum potassium is a poor proxy for the intracellular or whole-body potassium concentration (Blumberg et al., 1997). Indeed, serum potassium barely changes even when vast amounts of bananas are ingested, even though bananas are enriched in potassium, as will be outlined later in this article (Miller, 1997). Before the emergence of dual-energy X-ray absorptiometry, the determination of whole-body potassium content in this manner was also used for determining gross body composition based on empirical equations that took advantage of the fact that most of the body’s potassium, approximately 80%, is concentrated in the intracellular compartment of skeletal muscle. Meanwhile, ~18% is mainly present in the intracellular compartment of other tissues and only the remaining few % is present in the extracellular space (Forbes et al., 2000).
A radioactive trace of our supernova past
The stellar origin of the 40K present throughout the Solar System, including our bodies, is described in the so-called “supernova trigger hypothesis”. Accordingly, the Solar System was created 4.6 billion years ago from a molecular gas cloud that consisted mostly of hydrogen and helium created in
the immediate aftermath of the Big Bang almost 10 billion years earlier (Cameron and Truran, 1977; Podosek et al., 2000). However, the gas cloud also contained isotopes of heavier and mostly stable elements that had been created by stellar nucleosynthesis in already ancient stars, which had spread them by bursting into supernovae. Of note, these elements included the stable potassium isotopes 39K and 41K. It was the shockwave from a nearby type II supernova (Fig.2), resulting from the core collapse and concomitant explosion of a star with a mass of 8 to 50 times that of the Sun, that caused the presolar molecular gas cloud to collapse into a disc with a protostar at the centre. This would eventually become the Solar System as we know it.
With the shock wave, also followed an ejecta containing elements created within the type II supernova, including 40K, which was diluted within the existing potassium isotope pool, so that it initially made up 0.15% of the potassium. Due to its continuous decay over the past 4.6 billion years, it now makes up 0.0117% of potassium, while the stable isotopes 39K and 41K make up the remaining 93.1% and 6.88%, respectively. The healthy human body contains somewhere between
140 and 180 g of potassium (2.2–2.8 x 1024 potassium atoms), and thus ~0.2 g 40K, the decay of which continuously produces between 4000 and 5000 disintegrations per second. This makes 40K the single largest naturally occurring internal source of radiation in humans, responsible for approximately 5% of the annual natural background radiation in the UK, which is ~2.5 mSv.

Discussing 40K in the physiology classroom: a pop culture rendezvous
So, I am very fond of using case examples from pop culture when I give lectures in physiology (Berg, 2019). When I talk about electrolyte homeostasis and mention 40K, it has happened more than once that an excited student has asked whether it is because of this isotope that Dr. Manhattan, from the acclaimed graphic novel Watchmen, emanates a blue glow from his skin. This is a feature he obtains after being trapped in a so-called “intrinsic field subtractor”, which disintegrates him entirely on a subatomic level. After this, he somehow reassembles, and apart from his conspicuously glowing skin he then gains the ability to exist outside space and time. This would be a great story to pull off in the classroom, but unfortunately, this has nothing to do with 40K! Firstly, if it were true, we would then primarily be seeing a blue glow from Dr. Manhattan’s skeletal muscle and not his skin.
Secondly, the decay of 40K does not emanate a blue glow. According to an interview with James Kakalios, who is a physics professor at the University of Minnesota and a pioneer of using pop culture in communicating science, the blue glow reflects so-called Cherenkov radiation (Rogers, 2009), which occurs during the decay of certain radioactive isotopes that leak high-energy electrons that travel faster than the speed of light in a given medium.
During one of the abovementioned lectures where Dr. Manhattan was brought up, I mentioned that it may be the isotope 90Sr that gives off Cherenkov radiation, and that it may somehow have replaced the calcium in Dr. Manhattan’s body, since these elements have similar chemical attributes. A very bright student then remarked: “Ah, that would make it a bone seeker – that must be the reason Dr. Manhattan’s bones are shown so clearly through the skin on The Physiological Society’s website.” Now, I do always recommend my students to look up The Physiological Society online and to follow us on social media, and kudos to this student for actually doing so, especially while also thinking critically about the things I say, even when I’m just trying to be a bit funny. I did have to look up what the student meant; and truly so, on our website, there is an avatar-like man with transparent bluish skin. He could pass for Dr. Manhattan any day (Fig.3).
Going bananas for an index of radiation exposure
Given that radioactivity is inherently linked to superhero powers, particularly in comics from the 1960s and 1970s, I have previously attempted to highlight Bananaman as a much more useful example of how 40K may play into understanding phenomena in pop culture while also conveying some fundamental radiation biology with relevance for electrolyte homeostasis. However, while Bananaman is certainly a cult figure in the UK, he clearly does not have the same bad boy appeal as Dr. Manhattan, at least not here in Denmark. When I explained how he is a schoolboy who transforms into a caped (anti)hero when he eats a banana, and then gets a very diverse set of skills, ranging from intense stupidity to super strength (these two are not mutually exclusive in the Bananaman franchise), students just gazed at me with a blend of disappointment and distrust. My idea was to use this narrative as a vehicle for going into the concept of banana equivalent dose (BED), but since then I have just skipped the Bananaman part. Perhaps it will work better in the UK where he is better known and has a somewhat wider appeal.
But in terms of BED, the concept is that bananas have a high content of potassium, which is only outmatched by certain tropical fruits, such as avocado, guava and dates. In a Western diet, bananas are one of the major sources of dietary potassium along with potatoes, kidney beans, and nuts (Lanham-New et al., 2012). A 150 g banana contains approximately 0.54 g of potassium, and thus 63 x 10-6 g of 40K. Thus, eating one average sized banana gives rise to a radiation dose of 0.1 μSv. This has thus been coined BED, an informal unit of radiation exposure. One BED corresponds to approximately 1% of the average daily background radiation exposure; a flight from London to New York corresponds to 320 BED, a chest X-ray up to 1,000 BED, and a chest CT to 70,000 BED. However, while BED may be used for general educational purposes, it is a flawed measure in practice that is neither used clinically nor in research (i.e. hits on PubMed on 29 January 2023: 0). This is because the radiation dose from banana ingestion is not cumulative, and given that whole-body potassium is tightly regulated to maintain homeostasis, any excess potassium is excreted through the kidneys within a few hours after ingestion (McDonough and Fenton, 2022).
Concluding your journey through space and time
The understanding of potassium homeostasis is paramount for understanding almost all areas of physiology, and for grasping the complexity of the mechanisms at play in various potassium disorders in the clinical setting. Here, I have taken you on a journey through space and time with 40K, the isotope that is omnipresent albeit in miniscule amounts, and I hope that apart from reflecting on the fact that “we are stardust”, you will think a little about how many bananas you theoretically need to spare next time you go for an overseas flight, and that you also have a renewed taste for revisiting Joni Mitchell, Watchmen, and (perhaps) Bananaman.
References
Berg RMG (2018). Using Case Studies from Popular Culture to Teach Medical Physiology. Handbook of Popular Culture and Biomedicine: Knowledge in the Life Sciences as Cultural Artefacts (Eds: Gorgen A, Nunez A, Fangerau H). Springer, pp. 307-319.https://www.springer.com/gp/book/9783319906768
Bin Samat S, Green S, Beddoe AH (1997). The 40K activity of one gram of potassium. Physics in Medicine and Biology 42, 407-413. https://doi.org/10.1088/0031-9155/42/2/012
Blumberg A, Roser HW, Zehnder C, Muller-Brand J (1997). Plasma potassium in patients with terminal renal failure during and after haemodialysis; relationship with dialytic potassium removal and total body potassium. Nephrology Dialysis Transplantation 12, 1629-1634. https://doi.org/10.1093/ndt/12.8.1629
Cameron AGW, Truran JW (1977). The supernova trigger for formation of the Solar System. ICARUS 30, 447-461. https://doi.org/10.1016/0019-1035(77)90101-4
Forbes GB, Griggs RC, Moxley RT 3rd, Thornton CT, Tawil R (2000). K-40 and dual-energy
X-ray absorptiometry estimates of lean weight compared. Normals and patients with neuromuscular disease. Annals of the New York Academy of Sciences 904, 111-114. https://doi.org/10.1111/j.1749-6632.2000.tb06431.x
Lanham-New SA, Lambert H, Frassetto L (2012). Potassium. Advances in Nutrition 3, 820-821. https://doi.org/10.3945/an.112.003012
McDonough AA and Fenton RA (2022). Potassium homeostasis: sensors, mediators, and targets. Pflügers Archiv – European Journal of Physiology 474, 853-867. https://doi.org/10.1007/s00424-022-02718-3
Miller KC (2012). Plasma potassium concentration and content changes after banana ingestion in exercised men. Journal of Athletic Training 47: 648-654.https://doi.org/10.4085/1062-6050-47.6.05
Podosek FA, Podosek FA et al. (2000). Potassium, stardust, and the last supernova. Geochimica et Cosmochimica Acta 64, 2351-2362. https://doi.org/10.1016/S0016-7037(00)00359-8
Rogers V. Watchmen: The Science of Dr. Manhattan [Interview with James Kakalios]. https://www.livescience.com/3356-watchmen-science-drmanhattan.html. Published March 6, 2009.
