
Physiology News Magazine
A chemical engineering perspective of the gut
The flow patterns existing within the intestine are a function of wall motility and the flow properties of digesta, and are likely to affect the efficiency of nutrient uptake. Spatiotemporal mapping of intestinal motility and chemical engineering techniques are being used to evaluate the physiology of gastrointestinal mixing
Features
A chemical engineering perspective of the gut
The flow patterns existing within the intestine are a function of wall motility and the flow properties of digesta, and are likely to affect the efficiency of nutrient uptake. Spatiotemporal mapping of intestinal motility and chemical engineering techniques are being used to evaluate the physiology of gastrointestinal mixing
Features
Patrick W M Janssen & Roger G Lentle
Institute of Food, Nutrition and Human Health, Massey University, Palmerston North, New Zealand
https://doi.org/10.36866/pn.71.37

The form and function of the gut are likely to be limited by the same fluid dynamic constraints that limit the efficiency of chemical reactors (Penry & Jumars, 1986). However, the interplay between contractile events in the wall of the intestine and the rheological properties of digesta and their influence on the efficiency of mixing and absorption are poorly understood. The use of quantitative techniques to assess mixing that are based on those developed by chemical engineers in conjunction with simultaneous longitudinal and radial high resolution spatiotemporal mapping are starting to unravel the complex dynamics of mixing in the living gut.
The efficient absorption of nutrients requires the movement of nutrients from the lumen to the interior of the enterocyte. Two processes contribute to this movement: radial mixing (i.e. forced convection) and the slower molecular diffusion (Edwards et al. 1988). Regardless of the absorptive efficiency of the enterocyte, the rate of uptake of a nutrient from the lumen will be compromised if the radial mixing is insufficient to maintain high nutrient concentrations adjacent to the enterocyte. Radial mixing is best achieved within the lumen of the gut by the establishment of turbulent flow. Nutrient transport may be slower within the mucus layer as it may be influenced by molecular diffusion, and hence, a nutrient concentration gradient will exist between the periphery of the lumen and the surface of the enterocyte.
The pattern of flow of a liquid such as water through a simple tube is generally laminar at velocities similar to the average rate of flow through the gut. Laminar patterns of flow are characterised by a concentric series cylinders of fluid flow of increasing velocity from the wall to the centre of the lumen which travel in parallel and therefore are not conducive to efficient radial mixing. The disrupt-ion of this laminar pattern requires elements of the liquid to have sufficient kinetic energy, the level of which depends upon the relative magnitudes of the viscous and inertial forces exerted on it, i.e. the Reynolds number (Re). Hence, in laminar flow, Re is low and viscous forces predominate. When Re is high, inertial forces dominate and elements of fluid will have sufficient kinetic energy to form turbulent eddies each of widely differing length and duration.
While it is possible to predict the extent of turbulence and radial mixing of material in simple tubular conduits using the rheometric characteristics of digesta, the modelling of flow in the intestine is rendered complex by its gross and microscopic morphologies, and the timing and form of the various types of contractile activity. However, the type of flow regime that prevails in a reactor system can be inferred from the concentration profile of a pulse of dye marker after it has traversed that system. We applied this technique to measure the extent of mixing in living isolated segments of small intestine (Janssen et al. 2007).

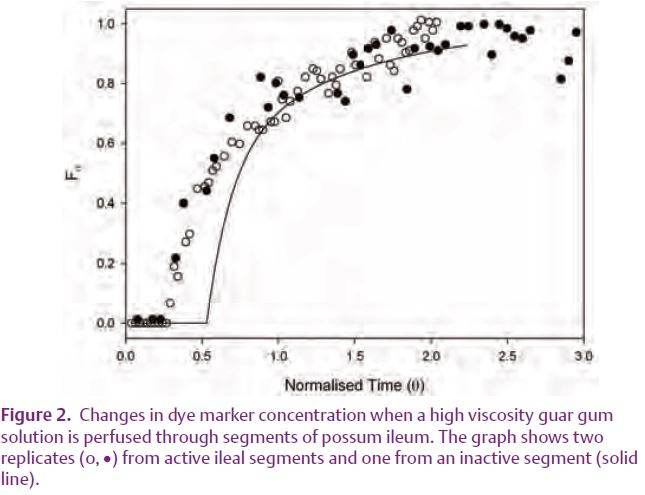
Active segments perfused with low viscosity solutions displayed a pattern of marker distribution characteristic of strong turbulence with chaotic vortices of widely different lengths and durations (Fig. 1). This indicated that efficient radial mixing could be readily established; a finding that differed from that predicted by modelling the flow of a watery liquid through a simple conduit. When segments were perfused with a guar gum solution with similar flow properties to those of distal small intestinal digesta i.e. a high apparent viscosity that is reduced by shear (pseudoplasticity), the marker distribution indicated a laminar mixing regime (Fig. 2). This laminar mixing regime was less effective than turbulence at generating mixing in both longitudinal and radial directions. Hence, while radial mixing is widespread in watery digesta such as may enter the proximal gut, it is less evident in more viscous pseudoplastic digesta such as is found in the more distal small intestine, hence the latter may require longer transit time to enable adequate nutrient absorption to occur.
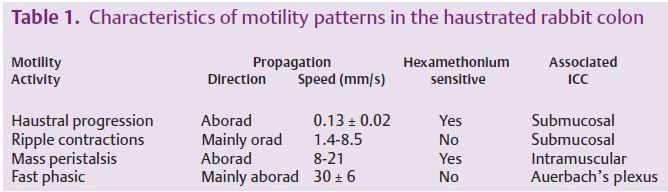
This work indicates the importance of motility in determining the flow regime and efficiency of nutrient uptake in the intestine. The study of motility in isolated sections of intestine has been furthered by the development of a spatiotemporal mapping technique that condenses circular muscle motility from a sequence of images onto a 2-dimensional map (Hennig et al. 1999). Our group has developed a mapping technique based on cross-correlating sequential images that also allows the visualisation of longitudinal muscle motility (Lentle et al. 2007b). These techniques were used to show that the turbulence obtained with low viscosity perfusates was due to highly oscillatory flow within the segment. While the average velocity of flow in the segment was low, contractile activity was sufficient to locally increase flow rates and fluid inertia to a level that brought about widespread turbulence. The relative magnitude, the phase relationship and the interaction between patterns on the circular and longitudinal maps has been used to characterise differing contractile patterns in the proximal colon of the rabbit and to infer separate levels of organisation of interstitial cells of Cajal (Lentle et al. 2008) (Table 1).
Further work is under way using dye markers to characterise the mixing initiated by these differing contractile activities.
Aside from providing a greater understanding of the physiology of gastrointestinal transit and mixing, these findings have significant implications for nutritionists and pharmacologists. A detailed understanding of the effects of the rheometric properties of food ingredients on the efficiency of mixing and absorption will aid in the development foods that are designed to impair or promote the absorption of macronutrients, or in the targeting of pharmaceutical agents at the distal gut mucosa. Moreover, they afford sensitive ex-vivo models that are convenient for determining the effects of pharmaceuticals that are known to influence motility and mixing.
References
Edwards CA, Johnson IT & Read NW (1988). Do viscous polysaccharides slow absorption by inhibiting diffusion or convection? Eur J Clin Nutr 42, 307-312.
Hennig GW, Costa M, Chen BN & Brookes SJH (1999). Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol 517.2, 575-590.
Janssen PWM, Lentle RG, Asvarujanon P, Chambers P, Stafford KJ & Hemar Y (2007). Characterisation of flow and mixing regimes within the ileum of the brushtail possum using residence time distribution analysis with simultaneous spatio-temporal mapping. J Physiol 582, 1239–1248.
Lentle RG, Janssen PWM, Asvarujanon P, Chambers P, Stafford KJ & Hemar Y (2008). High-definition spatiotemporal mapping of contractile activity in the isolated proximal colon of the rabbit. J Comp Physiol B178, 257–268.
Lentle RG, Janssen PWM, Asvarujanon P, Chambers P, Stafford KJ & Hemar Y (2007). High definition mapping of circular and longitudinal motility in the terminal ileum of the brushtail possum Trichosurus vulpecula with watery and viscous perfusates. J Comp Physiol B177, 543-556.
Penry DL & Jumars PA (1986). Chemical reactor analysis and optimal digestion. Bioscience 36, 310-315.
