
Physiology News Magazine
A little bit of ammonium may be good for your brain
Although specific ammonium-transporting proteins are well known in bacteria and plants, only recently has molecular and functional evidence begun to accumulate for ammonium transporters in animals. We describe how an ammonium transporter on the glial cells of bee retina plays an essential role in energy metabolism, and suggest an update of an old idea of a neuron-astrocyte flux of ammonium at synapses in mammalian brain
Features
A little bit of ammonium may be good for your brain
Although specific ammonium-transporting proteins are well known in bacteria and plants, only recently has molecular and functional evidence begun to accumulate for ammonium transporters in animals. We describe how an ammonium transporter on the glial cells of bee retina plays an essential role in energy metabolism, and suggest an update of an old idea of a neuron-astrocyte flux of ammonium at synapses in mammalian brain
Features
Païkan Marcaggi
Department of Neuroscience, Physiology and Pharmacology, University College London
Jonathan A Coles
Grenoble Institute of Neuroscience, France (present address: Centre for Biophotonics, University of Strathclyde, UK)
https://doi.org/10.36866/pn.73.15

Ammonium transporters are widespread
It is convenient to use the word ‘ammonium’ to include NH4+ (the ‘ammonium’ of chemists), NH3 (‘ammonia’) and the mixture of the two that spontaneously forms in water. At physiological pHs the equilibrium mixture contains about 98% NH4+ and 2% NH3 (see Marcaggi & Coles, 2001). NH3 can diffuse through lipid membranes (although not nearly as well as oxygen does). As shown by measuring intracellular pH, if you add NH4Cl outside a cell, NH3 entering the cell tends to alkalinize the cytoplasm because about 98% of the NH3 combines with H+ (Fig. 1). In practice, some ammonium goes in as NH4+, not NH3. A small fraction (about 2%) of this entering NH4+ dissociates to form NH3 and some H+ ions: the enteringflux of NH4+ would have to be more than about 50 times that of NH3 to produce a net acid shift. In most cells there is an initial alkalinisation caused by NH3 entry, then NH4+ entry produces a slow secondary acidification, but in some cells there is a primary acid shift, indicating that they take up overwhelmingly the NH4+ form (*1). The NH4+ ion is about the same size as a K+ ion and the conservative suggestion was that NH4+ was just leaking in via K+ channels or transporters. However, proteins of the Rh family (notorious for the immune reactions of Rh(-) foetuses to the blood of a Rh(+) mother) which are expressed not only in erythrocytes but also in kidney, liver, testes and parts of the brain, have homology with the Amt family of ammonium transporters, found in apparently all organisms up to and including invertebrates (Huang & Peng, 2005).
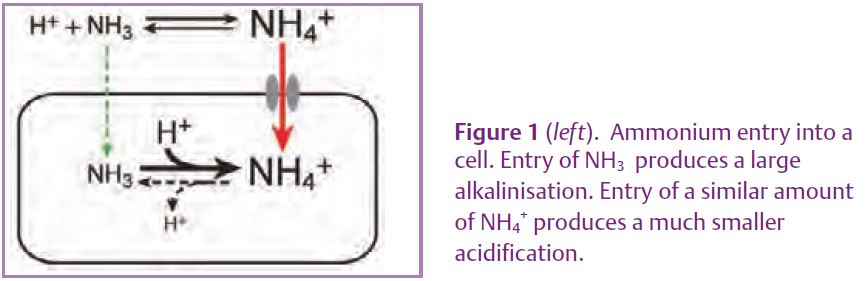
*1: It is easy to get the textbook alkaline response: you simply use a damaged cell with an unphysiologically low intracellular pH.
There is general agreement that Amt and Rh proteins have an external binding site for NH4+, but lively debate about what happens next. One possibility is that a proton is stripped off NH4+ to leave NH3 which passes through a channel, but there are also reports of electrogenic transport, i.e. transport of NH4+ orcotransport of NH3 and H+ (Javelle et al. 2008). Surprisingly, although Amt/Rh proteins are so widespread, the first ammonium transport selective for NH4+ over K+ to be demonstrated in an animal cell appears to be by another, molecularly different variety of ammonium transporter found in the honeybee retina.
A NH4+-selective cation-Cl- co-transporter on glial cells of the bee retina
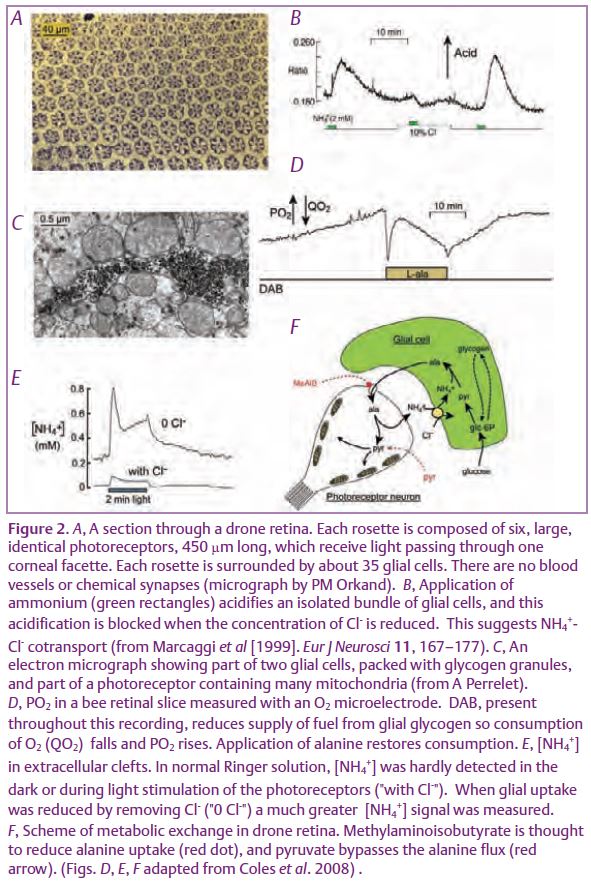
The drone (male) honeybee has a large eye, which is useful for spotting high-flying queens, and whose retina is composed almost exclusively of photoreceptor neurons and a homogeneous population of glial cells, arranged with impressive regularity (Fig. 2A). When the intracellular pH of retinal glial cells is measured with a pH-sensitive fluorescent dye, application of ammonium is found to produce a robust intracellular acidification that is abolished in the absence of Cl- (Fig. 2B). This suggests co-transport with Cl-. The transporter is selective for NH4+ over K+ (Marcaggi & Coles, 2000), but it shares with a wide range of K+-Cl- cotransporters the presence of inhibitory binding sites for bumetanide and piretanide.
These results suggest a NH4+-Cl-cotransporter which may be molecularly related to K+-Cl-cotransporters, but which is different from ammonium transporters of the Amt/Rh superfamily. The bee genome includes three Amt/Rh genes, thus making a minimum possible total of four ammonium transporters in this species.
NH4+-Cl- cotransport is central to energy metabolism in bee retina
A long series of papers have shown that, in drone retina, glucose is taken up exclusively by the glial cells, where it is converted to pyruvate then alanine. Tsacopoulos et al.(1994) proposed that the alanine is transferred to the photoreceptor neurons where it is deaminated to provide pyruvate for the numerous mitochondria (Fig. 2C), and that ammonium is returned to the glia, to support sustained production of alanine from glucose or glycogen. Two experimental tools not available to the earlier workers have recently been used to test and refine this model (Coles et al. 2008). First, it was not known whether these neurons can actually use alanine as a fuel. Normally, in a retinal slice, the huge stocks of glycogen in the glia provide a supply of fuel to the neurons that lasts for many hours. This supply can be reduced by DAB (*2), an inhibitor of glycogen phosphorylase, as can be seen by a fall in O2 consumption by the mitochondria (present only in the neurons). It was found that application of alanine restored O2 consumption, as predicted (Fig. 2D). Second question: do the photoreceptors release ammonium?Instead of trying to guess ammonium movements from measurements of pH, the authors used a triple-barrelled ammonium-sensitive microelectrode to directly measure [NH4+] in the extracellular clefts. In normal Ringer solution, extracellular [NH4+] was below the detection limit of these electrodes. However, when NH4+ uptake by the glia was blocked by removing Cl-from the Ringer solution, extracellular [NH4+] was measurable in the dark and shown to increase during light stimulation (Fig. 2E). Extracellular [NH4+] was greatly reduced by methylaminoisobutyrate, a blocker of amino acid transport, and also by pyruvate, which was expected to provide a direct substrate for the mitochondria, bypassing alanine (Fig. 2F). Hence, in this preparation, we now have very strong evidence for the neurons being fuelled by alanine, with ammonium being returned to the glial cells and taken up by a specific transporter.
*2: 1,4-Dideoxy-1,4-imino-D-arabinitol.
A neuron-astrocyte ammonium-amino acid shuttle in mammalian brain?
Mammalian astrocytes (at least in culture) avidly take up NH4+ (see Marcaggi & Coles, 2001). Most of the glutamate released at glutamatergic synapses in the mammalian brain is taken up into astrocytes by glutamate transporters where, in the astrocytes, it reacts with ammonium to form glutamine. Glutamine, which does not bind to synaptic receptors, is transferred back to the pre-synaptic terminals and deaminated to glutamate. By analogy with bee retina, the shuttle might be completed by transfer of ammonium from the neurons to the astrocytes, as originally proposed by Benjamin & Quastel (1975). Fig. 3, is an update of this proposal which shows uptake of NH4+ rather than NH3, and the co-release of ammonium with neurotransmitters (Marcaggi, 2006).
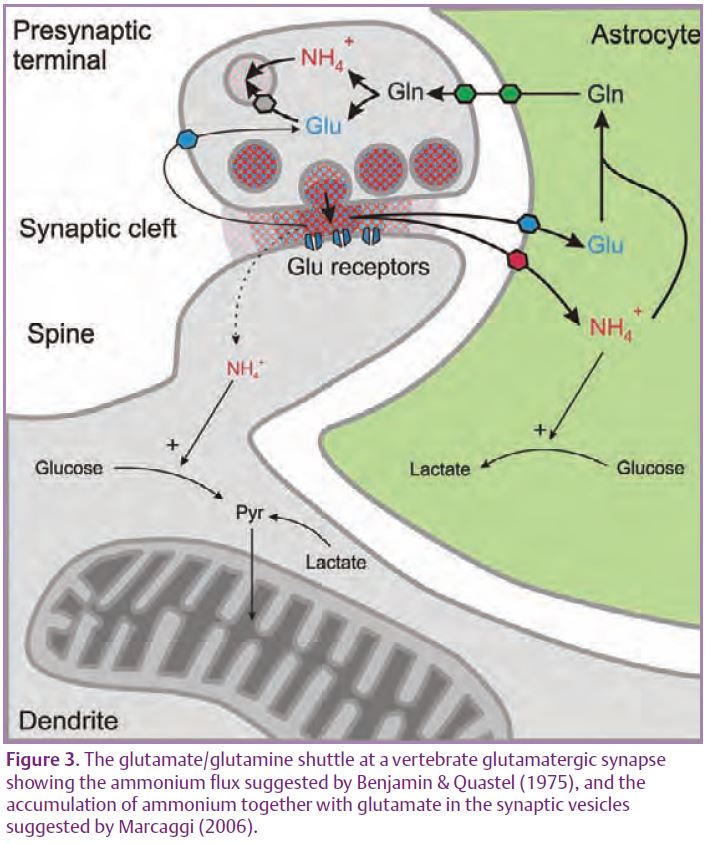
Within the astrocyte, ammonium might act as a signal as well as a reactant. For example, enzymes of the glycolytic pathway are stimulated by ammonium and exogenous ammonium does increase lactate production in rat brain in vivo (Provent et al. 2007). What would really help this field is a good membrane permeable ammonium-sensitive fluorescent indicator. Please tell your chemist friends.
References
Benjamin A & Quastel J (1975). Metabolism of amino acids and ammonia in rat brain cortex slices in vitro: a possible role of ammonia in brain function. J Neurochem 25, 197–206.
Coles JA, Martiel JL & Laskowska K (2008). A glia-neuron alanine/ammonium shuttle is central to energy metabolism in bee retina. J Physiol 586, 2077–2091.
Huang CH & Peng J (2005). Evolutionary conservation and diversification of Rh family genes and proteins. Proc Natl Acad Sci U S A 102, 15512–15517.
Javelle A, Lupo D, Ripoche P, Fulford T, Merrick M & Winkler FK (2008). Substrate binding, deprotonation, and selectivity at the periplasmic entrance of the Escherichia coli ammonia channel AmtB. Proc Natl Acad Sci U S A 105, 5040–5045.
Marcaggi P (2006) An ammonium flux from neurons to glial cells. Proc Physiol Soc 3:SA16.
Marcaggi P & Coles JA (2000). A Cl-cotransporter selective for NH4+ over K+ in glial cells of bee retina. J Gen Physiol 116, 125–141.
Marcaggi P & Coles JA (2001). Ammonium in nervous tissue: transport across cell membranes and fluxes from neurons to glial cells. Prog Neurobiol 64, 157–183.
Provent P, Kickler N, Barbier EL, Bergerot A, Farion R, Goury S, Marcaggi P, Segebarth C & Coles JA (2007). The ammonium-induced increase in rat brain lactate concentration is rapid and reversible and is compatible with trafficking and signaling roles for ammonium. J Cereb Blood Flow Metab 27, 1830–1840.
Tsacopoulos M, Veuthey AL, Saravelos G, Perrottet P & Tsoupras G (1994). Glial cells transform glucose to alanine which fuels the neurons in the honeybee retina. J Neurosci 14, 1339–1351.
