
Physiology News Magazine
A man is as old as his arteries: a scientific journey of ageing and aortic function
Arterial elasticity declines with age.
Features
A man is as old as his arteries: a scientific journey of ageing and aortic function
Arterial elasticity declines with age.
Features
Raya Al-Maskari & Yasmin
Division of Experimental Medicine & Immunotherapeutics, University of Cambridge, UK
https://doi.org/10.36866/pn.98.22
As the old adage goes; ‘there is more than meets the eye’. To put a spin on that; ‘there is more to the function of the aorta than meets the Heart’. As we age, our cardiac cells shrink in number but expand in size, which makes the heart wall thicker. Similarly, our arteries stiffen and this is associated with adverse conditions like hypertension, stroke, renal failure and heart disease. In this review, we aim to take the reader on a journey through the aorta’s multifaceted role and subsequently explore the degenerative effects of biological ageing on this organ.
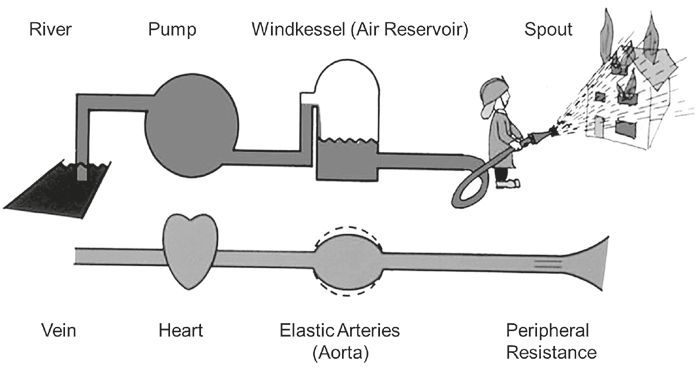
The aorta serves an important function as a conduit circulating blood to the peripheral organs from the left ventricle. A less recognized but equally fundamental function of the aorta is to act as a ‘cushioning’ chamber for the heart, dampening the high-energy pulsations generated by the heart beats and supporting the perfusion of organs. These functions have been traditionally exemplified by the Windkessel model, which was developed by the quantitative physiologist Otto Frank in 1899. The origin of the term comes from the German word for ‘air-chamber’. This was a water reservoir half-filed with air positioned behind the pumps of old fire hoses, which transformed the pulsing water output into a continuous stream (Fig. 1, Westerhof et al., 2009). With this picture in mind, it is easy to appreciate how the Windkessel model of the arterial system allows for an unremitting blood flow to the periphery by storing 50% of the stroke volume during the systolic phase of the heart beat and propelling it to the peripheral circulation during diastole, thus ensuring sustained perfusion of organs throughout the cardiac cycle.
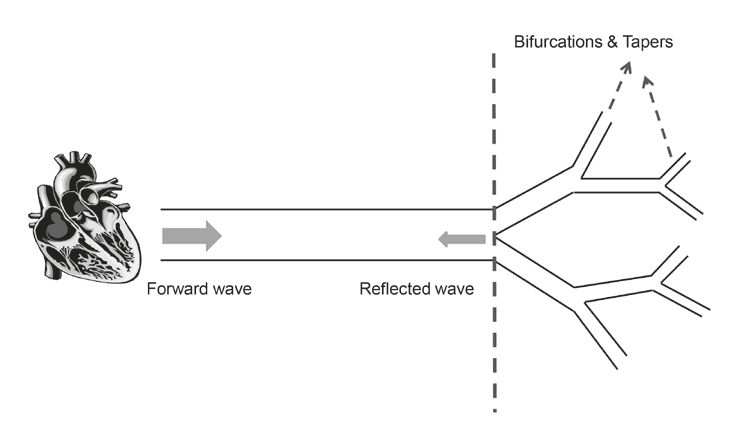
The function of the aorta, and its dysfunction, can further be illustrated by the aortic pressure waveform model. When the heart contracts, the pulse of blood ejected is accompanied by a pressure wave that originates from the ventricle walls. This pressure is transmitted in the forward direction, travelling away from the heart in waves at a speed known as the pulse wave velocity (PWV). The journey that the forward wave takes through the circulation is far from a straightforward one, as the propagating wave confronts many points of ‘mismatch’ along its path. The bifurcations and taperings of the circulatory system result in wave reflections that travel in an opposing direction to that of the forward wave, in other words, towards the heart and against the direction of blood flow (Fig. 2). In a healthy, compliant system where both cardiac and vascular events are meticulously synchronised, the reflected wave returns to the central aorta during late systole and early diastole giving rise to a secondary augmentation pressure at the aortic root (Shirwany and Zou, 2010). The precise timing of the return of the reflected wave with respect to the cardiac cycle is critical for the efficiency of the cardiovascular system. It allows for the augmentation of diastolic blood pressure, thereby enhancing coronary blood flow whilst ensuring that no additional pressure is produced during systole (O’Rourke, 2007).
Pulse wave velocity is related to arterial elasticity
PWV was recognised in 1922 as a marker of arterial elasticity and a fundamental index of ‘circulatory efficiency’ by Bramwell and Hill (Bramwell and Hill, 1922) but the notion of arterial wave analysis and the assessment of arterial pulse has been known since the late Han dynasty (Parker, 2009) when one of the first books ‘the Pulse Classic’, was written in 220 AD. Moreover, the concept of forward pulse wave transmission was identified by Erasistratos as early as 280 BC, and he found that the pulse appeared earlier in arteries proximal to the heart than in those distal to the heart (Skalak et al., 1981). The field has progressed significantly since then from being a qualitative into a quantitative discipline particularly highlighting the haemodynamic and mechanical behaviour of the circulation. Aortic PWV is the current gold standard measure of arterial stiffness, measured non-invasively between any two arterial sites (i.e. carotid and femoral arteries or carotid and radial arteries). It increases with age, and typically in a 20 year old adult, it is 5m/s, whilst in an elderly person of 80 years it is 12m/s.
Role of Elastin and Collagen
The capacity of the artery to contract and expand to accommodate the cardiac cycle pressure oscillations is central to its function. The aortic wall is indeed viscoelastic in nature. This characteristic is the product of an intricate interplay of two structural proteins of the extracellular matrix, elastin and collagen. These two proteins are remarkably different. Elastin is 70-100% extensible, whilst collagen is only 2-4%; the latter is 1000 times stiffer than the former when both are stretched (Dobrin, 2000). The distinct contribution of these proteins to the mechanical behaviour of the aortic wall was initially demonstrated in the mid 1900s by Roach and Burton, in arterial samples in response to varying levels of pressure in humans. They found that elastin fibres are load-bearing at low pressures, while collagen fibres are predominantly load-bearing at high pressures. In other words, elastin is responsible for the compliance and structural integrity of arteries at low physiological pressures, whereas collagen imparts the tensile strength to arteries at higher physiological pressures. As such, any change in the collagen to elastin ratio, could detrimentally compromise the artery’s ability to accommodate changes in pressure. Indeed, this by-and-large, is the hallmark of arterial ageing.
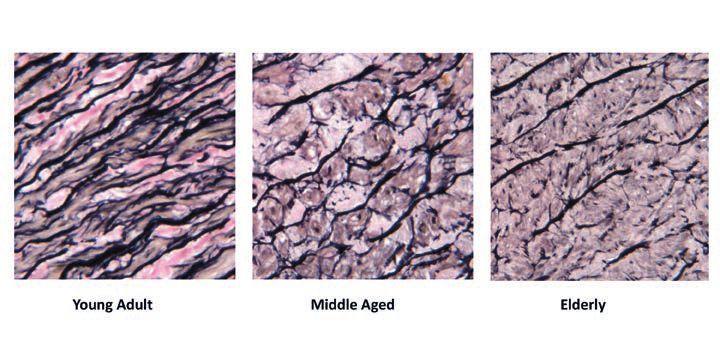
The arterial changes observed in ageing could be compared to a rubber band that has been stretched continuously for many years. Conceptually similar to the ‘wear and tear’ of rubber due to repetitive cycles of stretch and recoil, the cyclic stress and strain of arterial elastin leads to its ‘fracture and fatigue’. This results in the fundamental degenerative process seen in arterial ageing: fraying and fragmentation of elastin fibres (O’Rourke, 2007). The fracturing of rubber is estimated to occur after 1 x 109 oscillations, in cardiac time this is equivalent to 60-70 beats per minute over the course of 25-30 years in humans (Dobrin, 2000). Theoretically, this is the age when aortic elastin fibres begin to fragment. It is for this reason that the process of arterial degeneration has been said to commence in childhood and to be ‘well developed’ by early adulthood (Nichols et al., 2011). This stark difference in elastin fibre composition is clearly seen in Fig. 3. A number of structural and cellular modifications to the arterial wall further exacerbate the stiffening process. These include an increase in collagen content, calcium deposition, extracellular matrix (ECM) accumulation, increased vascular smooth muscle cell proliferation, reduced endothelial function and the intima-medial layer thickening (Park and Lakatta, 2012). Together, these lead to the dilatation, stiffening and thickening of arterial walls. The aforementioned degenerative changes are more prominent in the proximal large central arteries (i.e., aorta and its major branches) which have a higher elastin content and receive most of the impact of repeated cyclic strain and stress compared to the peripheral muscular arteries (i.e., radial).
Arterial Stiffness: The Impact
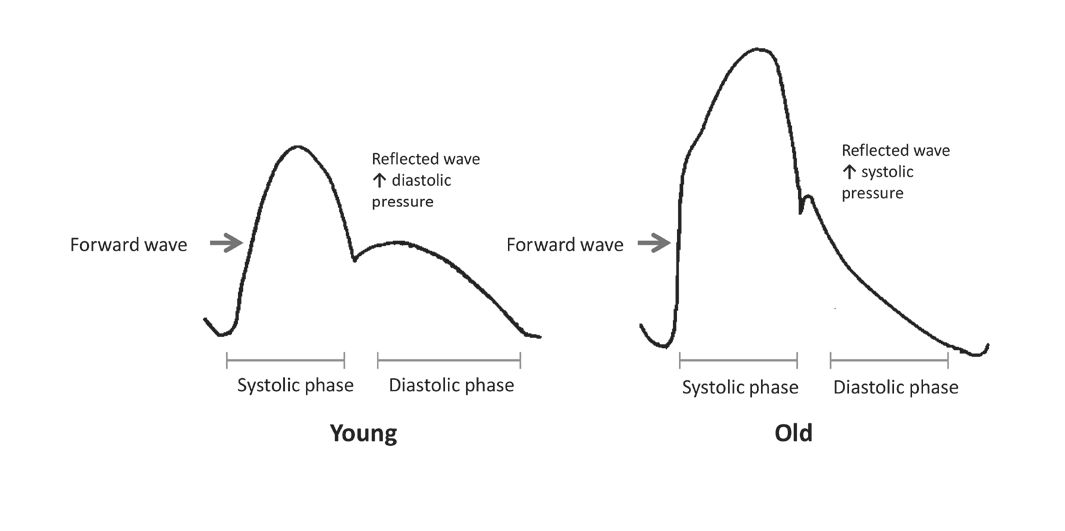
Arterial stiffening is detrimental in more ways than one. The fraying of elastin fibres means that the mechanical load is now shifted to the more rigid collagen component of the artery, compromising the cushioning function of the aorta and causing major changes to the aortic pressure waveform. When the central arteries lose cushioning efficiency the pressure pulsations travel further down the arterial tree into the vessels of the microcirculation triggering microbleeds and microinfarcts, thus increasing the risk of stroke, cognitive impairment and renal failure (O’Rourke and Hashimoto, 2007). With respect to the changes in the aortic pressure waveform, a stiffened artery causes an early return of the reflected wave such that it falls within the systolic phase instead of the diastolic phase (Fig. 4). The early return of reflected wave, coupled with a stiffened aorta, leads to a rise in systolic pressure and fall in diastolic pressure (O’Rourke and Hashimoto, 2007), giving rise to isolated systolic hypertension. The raised systolic pressure also causes an increase in left ventricular load driving left ventricular hypertrophy and increased cardiac oxygen demand (O’Rourke and Hashimoto, 2007). On the other hand, the reduced diastolic blood pressure makes the heart incapable of meeting this demand due to the compromised coronary perfusion, predisposing the heart to ischemia and, ultimately to myocardial infarction or heart disease (O’Rourke and Hashimoto, 2007).
Factors Regulating the Stiffening Process
As people age, arteries stiffen. However, it is not clear why arterial ageing and its associated complications manifest more often in some people than others. There is no straightforward answer to this question, as the mechanisms underlying age-related arterial stiffness are complex. Other than age, mean arterial pressure and smooth muscle tone regulate stiffness, whilst conditions such as diabetes, hypertension, high cholesterol and inflammation accelerate its progression (Horvath et al., 2014), thus accounting for some of the variability. The pathogenesis and progression of arterial stiffness is further governed by a complex network of molecular (e.g. gene expression), cellular (e.g. telomeres), biochemical and enzymatic pathways (e.g. matrix metalloproteinases) which alter the arteries capacity to adapt and repair in the face of the ageing process. Here, we focus briefly on one of the most important biomarkers, matrix metalloproteinase-9 (MMP-9), that is involved with elastin and collagen break down, and is associated with cardiovascular conditions and arterial stiffness.
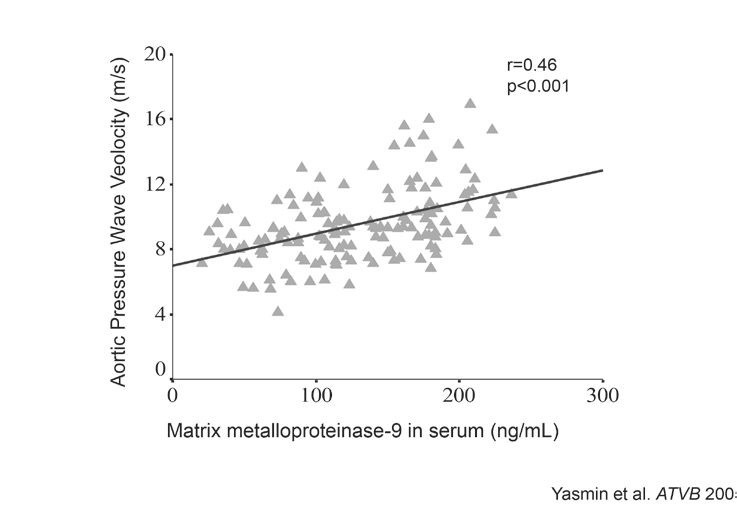
Matrix metalloproteinases (MMPs) are a group of proteolytic enzymes that have the capacity to catalyse the normal turnover of extracellular matrix (ECM) and also degrade its components like elastin and collagen. Although the balance between the matrix protein synthesis and degradation is tightly controlled by their inhibitors, as arteries age or undergo pathological changes, this balance is lost and MMP enzyme activity increases. We have previously demonstrated an association between serum MMP-9 levels and aortic stiffness in healthy individuals, and in isolated systolic hypertensives, indicating their role in aortic stiffness (Fig. 5, Yasmin et al., 2005). The exact role of MMPs in the stiffening process remains elusive, although current clinical and experimental data suggest an inflammatory mediated pathway, the discussion of which is beyond the scope of this article, but is well reviewed by Galis and Khatri, 2002.
Summary
The degenerative effect of ageing on the aorta is marked: structurally, it is manifested as fragmentation of elastin fibres rendering the aorta prone to remodelling and stiffening. Functionally, it is apparent as an increase in aortic PWV, which is a strong and independent predictor of cardiovascular events and all-cause mortality. Indeed, aortic stiffness underlies isolated systolic hypertension and contributes to the pathogenesis of stroke, dementia, left ventricular hypertrophy, renal damage and heart failure. Better elucidation of the mechanisms that drive the stiffening process could help to generate therapeutic targets that reverse or slow the impact of aging on aortic function.
References
Bramwell JC & Hill AV (1922). Velocity of transmission of the pulse-wave: and elasticity of arteries. The Lancet 199, 891-892.
Dobrin PB (2000). Elastin, Collagen and the Pathophysiology of Arterial Aneurysms. In: Keen RR & Dobrin PB (eds.) Development of Aneurysms. Georgetown, Texas: Landes Bioscience.
Horvath T, Osztovits J, Pinter A, Littvay L, Cseh D, Tarnoki AD, Tarnoki DL, Jermendy AL, Steinbach R, Metneki J, Schillaci G, Kollai M & Jermendy G (2014). Genetic impact dominates over environmental effects in development of carotid artery stiffness: a twin study. Hypertens Res 37, 88-93.
Lakatta EG (2008). Arterial aging is risky. J Appl Physiol (1985) 105, 1321-1322.
Nichols WW, O’Rourke MF & Vlachopoulos C (2011). McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. 6th ed. UK: Hodder Arnold.
O’Rourke MF (2007). Arterial aging: pathophysiological principles. Vasc Med 12, 329-41.
O’Rourke MF & Hashimoto J (2007). Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 50, 1-13.
Park S & Lakatta EG (2012). Role of inflammation in the pathogenesis of arterial stiffness. Yonsei Med J 53, 258-61.
Parker KH (2009). A brief history of arterial wave mechanics. Med Biol Eng Comput 47, 111-118.
Shirwany NA & Zou MH (2010). Arterial stiffness: a brief review. Acta Pharmacol Sin 31, 1267-1276.
Skalak R, Keller SR & Secomb TW (1981). Mechanics of blood flow. J Biomech Eng 103, 102-15.
Yasmin, Wallace S, McEniery CM, Dhakam Z, Pusalkar P, Maki-Petaja K, Ashby MJ, Cockcroft JR & Wilkinson IB (2005). Matrix metalloproteinase-9 (MMP-9), MMP2, and serum elastase activity are associated with systolic hypertension and arterial stiffness. Arterioscler Thromb Vasc Biol 25, 372-378.
Galis ZS & Khatri JJ (2002). Matrix Metalloproteinases in Vascular Remodeling and Atherogenesis. The Good, the Bad, and the Ugly. Circ Res 90, 251-262.
