
Physiology News Magazine
A new muscle sense?
Uwe Proske proposes that, whenever movement of our limbs is accompanied by an effort sensation, this information can be used by the brain to determine the location in space of the limbs
Features
A new muscle sense?
Uwe Proske proposes that, whenever movement of our limbs is accompanied by an effort sensation, this information can be used by the brain to determine the location in space of the limbs
Features
Uwe Proske
Department of Physiology, Monash University, Clayton, Victoria, Australia
https://doi.org/10.36866/pn.62.23
In a previous report (Physiology News 60, 14), I described our work on muscle exercise and how intense exercise of arm muscles, be it eccentric or concentric, can lead blindfolded subjects to make positional errors in a forearm matching task. The observations prompted us to propose that the muscle activity required to hold up the arm against the force of gravity provides us with information about where the arm is in space (Walsh et al. 2004). That is our new sense!
At the present time the generally accepted view is that muscle receptors, specifically the muscle spindles, provide us with our sense of limb position and movement. This view is firmly based on experimental observations. In 1972, Goodwin, McCloskey and Matthews showed that muscle vibration produced illusions of both movement and changed position of the forearm during vibration of elbow flexors (Goodwin et al. 1972). Since the primary endings of spindles were known to be selectively sensitive to vibration, the observations led to the present-day view that the kinaesthetic sense (position and movement) is generated by signals of a peripheral origin. Our observations, if confirmed, will require these ideas to be modified.
When we say that subjects derive spatial information from their sense of effort we mean that a certain amount of effort is required to hold the arm in a given posture against the force of gravity. If the arm is more horizontal, the gravitational vector will be larger, requiring more muscle activity to hold it there. The extra activity is perceived as more effort (Walsh et al. 2004). The actual relationship between effort and position is complicated by the fact that muscle force and torque at the elbow joint don’t change in parallel with change in angle, since the moment arm changes and muscle length changes too. In any case, we propose that for a given effort required to support the arm against gravity, the brain is able to derive information about its position in space. How the sense of effort is calibrated is another matter.
The idea that our muscle senses may have a central origin is not new. Von Helmholtz proposed a ‘sensation of innervation’ back in 1867. Since then the question of whether a proprioceptive signal is associated with the motor command has been repeatedly raised. For example, the present-day view of the sense of force or of heaviness is that it is derived centrally. The evidence is that force and heaviness matching can be disturbed by weakening the muscles with partial paralysis or fatigue. For a review, see (McCloskey et al. 1983).
As far as we know, it has not been specifically claimed before that the motor command can also provide positional information. In 1988 Peter Matthews declared, ‘… the interest now is not in asking simply whether corollary discharges are involved in the genesis of human position sense, for it seems to me that they must be’ (Matthews, 1988). By corollary discharges Matthews meant a copy of the central motor command. Be that as it may, there have been few specific proposals put forward which incorporate a central command signal in the perception of limb position. Thus Worringham and Stelmach (1985) concluded that torque sensation was an accessory source of information in limb positioning. Other reports have tended to interpret their observations in terms of an exclusively peripheral origin of the positional signals (Rymer & D’Almeida, 1980).
We propose that positional information from the sense of effort is available only when positioning of the limb is accompanied by muscle contraction. Does that mean the sense of effort plays a role in kinaesthesia only when the limb is held against the force of gravity? We think not. We are coming to the view that for all limb placements that involve muscle contraction, even in circumstances which are gravityneutral, such as moving the arm in the horizontal plane, effort plays a role in position sense.
What happens when there is no muscle activity and the muscle remains relaxed? If a blindfolded subject places their arm at a set angle on a support and the arm remains relaxed, position sense is rather poor, with subjects routinely making large matching errors (Winter et al. 2005). However, as soon as they support their arm themselves, their matching performance improves significantly (Fig. 1).
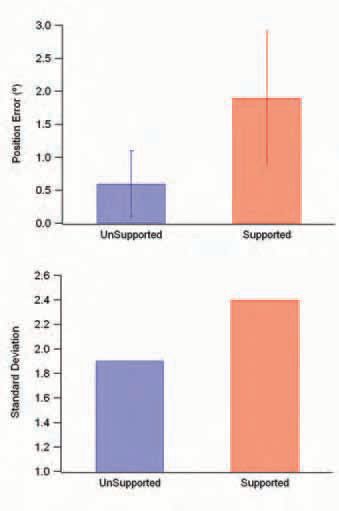
In another experiment we have been able to provide evidence that the crude position signal available to subjects when their arm is relaxed is coming from muscle spindles (Winter et al. 2005). The evidence is based on the observation that conditioning the muscle at different lengths leads subjects to make systematic errors in position sense (Fig. 2). The reason for this pattern of errors is that the contraction has conditioned the responses of the muscle spindles.

When a muscle is contracted voluntarily, both skeletomotor and fusimotor neurones are co-activated (Vallbo, 1974). This means that the intrafusal muscle fibres of spindles contract. If that is done in a posture where the muscle is short, and afterwards the muscle is stretched to an intermediate length, the intrafusal fibres will be taut and spindle resting discharge levels will be high. Alternatively, the muscle is contracted while holding the limb in a posture where the muscle is long. On moving it to a shorter length, the intrafusal fibres will fall slack and therefore resting discharge will be low (Gregory et al. 1988). Such contraction history dependent changes in spindle activity are reflected in the observed errors in position sense (Fig. 2). This is a powerful argument for spindles as the receptors responsible since other sensory receptors that might contribute, skin and joint receptors, are unlikely to show such history-dependent behaviour. Effort cannot play a role since the muscle has remained relaxed.
So, to summarise up to this point, when our limb muscles are relaxed, positional information is coming from muscle spindles. However, as soon as the task requires some muscle contraction, additional information from another source begins to contribute. We claim that this source is the sense of effort, generated centrally in association with the motor command.
Our current area of research concerns the changes in position sense observed when the limb is bearing a load. As already mentioned, during a voluntary contraction spindles become coactivated. The fusimotor activity might be expected to produce a sudden change in the position signal. Yet the observations are of a smooth transition from passive position matching to where the muscles are contracting (Fig. 2). We place particular importance on the observation that the conditioning effects gradually disappear as load increases. Fusimotor-activated spindles would not be expected to show muscle conditioning effects, since any slack introduced by conditioning would be taken up by the fusimotor activity (Gregory et al. 1988). During a contraction, while the effects of muscle conditioning are significantly less, for a submaximal contraction (15% of maximum in Fig. 2), some small conditioning effects persist. This suggests that the few remaining passive spindles are still able to manifest themselves as position errors.
What we are saying is that evidence of a spindle contribution to position sense in the passive muscle, and for low levels of contraction, is clearly discernible. Then, as the contraction grows, a new influence becomes apparent. Our experiments suggest it is the sense of effort. In an alternative view, spindles continue to provide the position signal but after some kind of central processing of their input, where fusimotor-related activity is subtracted
out (McCloskey et al. 1983). To resolve this issue is a challenge for the future.
References
Goodwin GM, McCloskey DI & Matthews PB (1972). The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain 95, 705-748.
Gregory JE, Morgan DL & Proske U (1988). Responses of muscle spindles depend on their history of activation and movement. Prog Brain Res 74, 85-90.
Matthews PBC (1988). Proprioceptors and their contributions to somatosensory mapping: complex messages require complex processing. Can J Physiol Pharm 66, 430-438.
McCloskey DI, Gandevia S, Potter EK & Colebatch JG (1983). Muscle sense and effort: motor commands and judgements about muscular contractions. In Motor Control Mechanisms in Health and Disease, ed. Desmedt JE, pp. 151-167. Raven Press, New York.
Rymer WZ & D’Almeida A (1980). Joint position sense. The effects of muscle contraction. Brain 103, 1-22.
Vallbo A (1974). Human muscle spindle discharge during isometric voluntary contractions. Amplitude relations between spindle frequency and torque. Acta Physiol Scand 90, 319-336.
Walsh LD, Hesse CW, Morgan DL & Proske U (2004). Human forearm position sense after fatigue of elbow flexor muscles. J Physiol 558, 705-715.
Winter J, Allen T & Proske U (2005). Muscle spindle signals combine with the sense of effort to indicate limb position. J Physiol 568, 10351046.
Worringham CJ & Stelmach GE (1985). The contribution of gravitational torques to limb position sense. Exp Brain Res 61, 3842.
