Physiology News Magazine
A novel feature of epithelial acetylcholine in intestinal luminal sensing
Textbooks describe acetylcholine (ACh) as a neurotransmitter that is released from cholinergic motor nerves in response to luminal stimuli to control gut motility and ion transport. In a new approach we demonstrated that ACh is synthesised in colonic epithelial cells and released on the basolateral side by luminal chemo-stimulation, which causes chloride secretion.
Features
A novel feature of epithelial acetylcholine in intestinal luminal sensing
Textbooks describe acetylcholine (ACh) as a neurotransmitter that is released from cholinergic motor nerves in response to luminal stimuli to control gut motility and ion transport. In a new approach we demonstrated that ACh is synthesised in colonic epithelial cells and released on the basolateral side by luminal chemo-stimulation, which causes chloride secretion.
Features
Takaji Yajima (1), Ryo Inoue (2), Megumi Matsumoto (1) and Masako Yajima (1)
1: Meiji Dairies Research Chair, Creative Research Institution, Hokkaido University, Sapporo, Japan
2: Laboratory of Animal Science, Kyoto Prefectural University, Kyoto, Japan
https://doi.org/10.36866/pn.83.26

Acetylcholine (ACh) is the best characterised neurotransmitter and is synthesised and stored in cholinergic neurons in the brain and gut wall. ACh synthesis is catalysed by choline acetyltransferase (ChAT) via the enzymatic conversion of acetyl-CoA and choline to ACh and CoA. Choline, the limiting substrate for ACh synthesis, is taken up by neurons by the high-affi nity choline transporter 1 (CHT1). Synthesised ACh is stored in synaptic vesicles by the vesicular acetylcholine transporter, and is released from nerve terminals by membrane action potentials in response to physiological and pharmacological stimuli.
The gut wall is richly innervated with cholinergic neurons, and ACh is a predominant neurotransmitter of the enteric nervous system (Harrington et al. 2010). When the cholinergic motor neurons are activated via an enteric refl ex by luminal stimuli (Fig. 1A), ACh is released at the neuro-enterocyte junction. Epithelial cells express many subtypes of muscarinic ACh receptors, which are involved in the refl ex secretory response to mechanical and chemical signals from the luminal side of the intestine (Raybould et al. 2004). Stimulating muscarinic ACh receptors induces mucosal hydration due to chloride secretion from the crypt cells and inhibition of sodium absorption from the apical cells, and stimulates mucus secretion from the goblet cells. Thus, intestinal cholinergic regulation plays a role in providing surface lubrication to propel luminal contents aborally or for fl ushing out harmful microbes and toxins.

It is also noteworthy that ACh is synthesised and stored in a broad variety of non-neuronal cells, particularly in surface epithelia of the airway, bladder, placenta and skin of rodents and humans (Wessler & Kirkpatrick, 2008). Synthesis and storage of ACh in non-neuronal cells has been confi rmed not only by positive anti-ChAT immunoreactivity but also by direct measurements with high performance liquid chromatography combined with a post column enzyme reactor and an electrochemical detector. ACh is not stored in vesicles of non-neuronal cells and its release is mediated by organic cation transporters (OCTs). Therefore, ACh release from non-neuronal cells is much slower than that from nerve terminals. Non-neuronal ACh also occurs in the epithelial cells of the small and large intestines of rats and humans (Klapproth et al. 1997).
However, little progress has been made regarding the physiological signifi cance of the synthesis and release of non-neuronal ACh into the intestine.
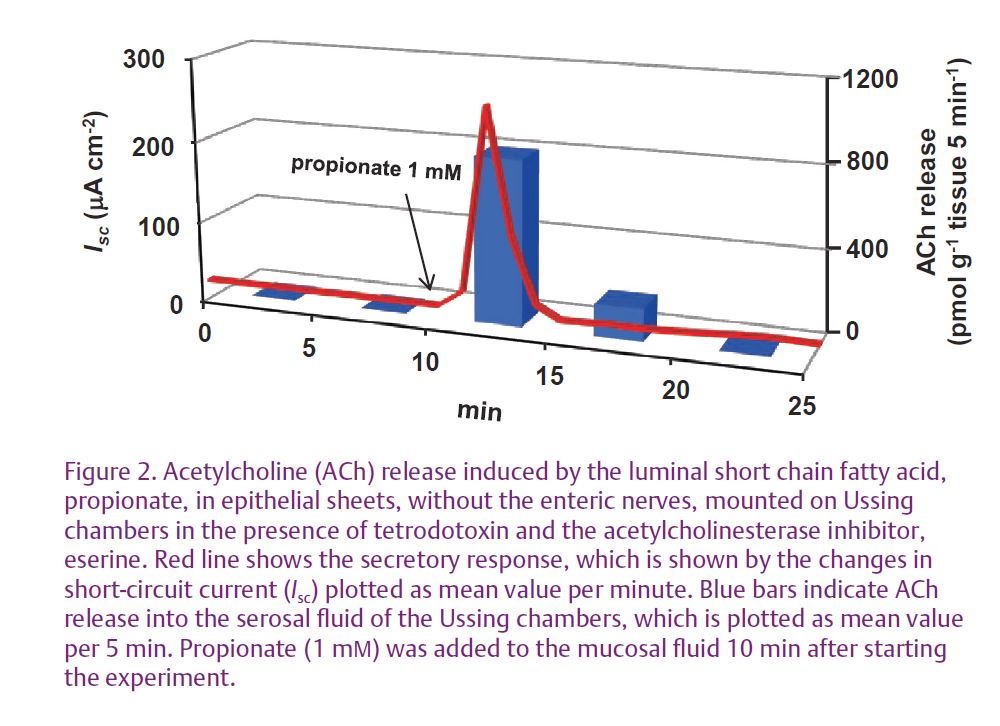
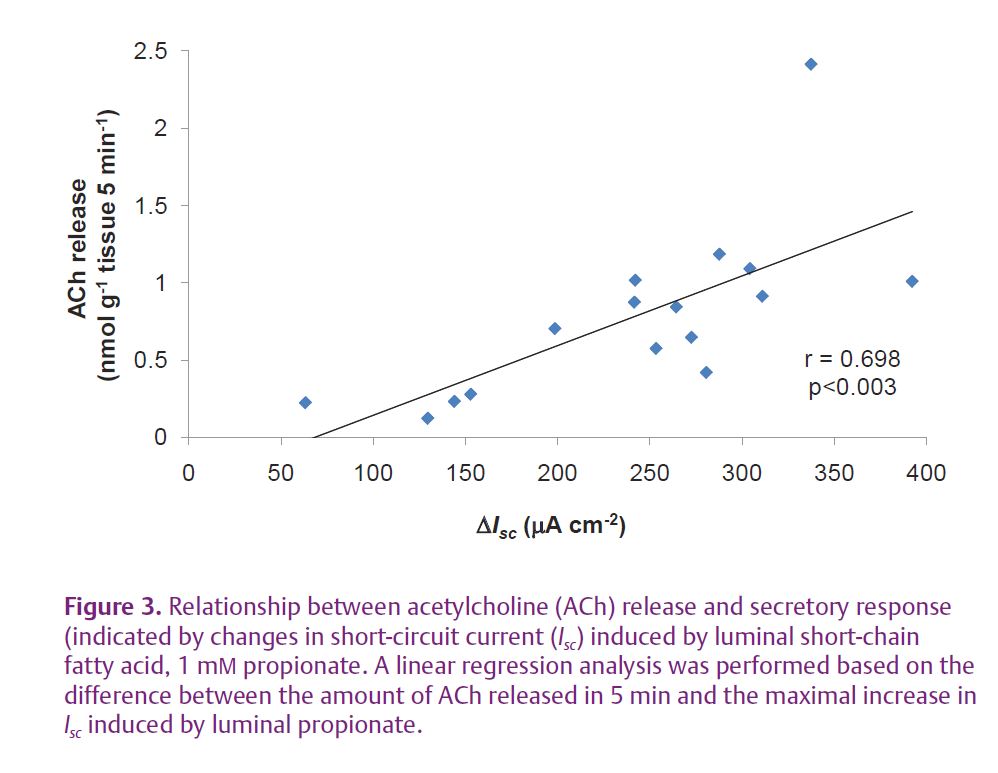
Previous studies have demonstrated that luminal short chain fatty acids (SCFAs) are major colonic microbial fermentation products and stimulate chloride secretion in the rat distal colon in vitro (Yajima, 1988). SCFA-stimulated chloride secretion is induced even under a nerve block by tetrodotoxin but is inhibited by atropine, a strong cholinergic receptor antagonist. Therefore, ACh may be released from cholinergic nerve terminals depolarised by an unknown signal substance (Fig. 1A) or released non-neuronally from colonic epithelial cells (Yajima, 1988; Hubel & Russ, 1993). In a recent study reported in The Journal of Physiology, we addressed the involvement of non-neuronal ACh in the secretory response to SCFA in rat colon (Yajima et al. 2011). Using epithelial sheets mounted on Ussing chambers that did not include the enteric nerve plexuses, we showed that the luminal stimulation by the SCFA, propionate, caused ACh release into the serosal side (Fig. 2). A signifi cant linear relationship was observed between ACh release and chloride secretion, as indicated by changes in short-circuit current (Fig. 3), suggesting that the release of ACh occurred non-neuronally and then caused chloride secretion by stimulating epithelial muscarinic receptors. These results were confirmed in an experiment showing that a muscarinic receptor antagonist and an inhibitor of chloride secretion inhibited chloride secretion but not ACh release.
To confirm the non-neuronal origin of ACh release in response to luminal propionate stimulation, we employed a method of crypt isolation from colonic segments to obtain neural components free of epithelial cells. We demonstrated the ChAT gene mRNA expression as well as the storage of a considerable amount of ACh compared to residual muscle tissues including the enteric nerves. Gene expression of OCTs but not CHT1 indicated that colonic epithelial cells use OCTs rather than CHT1 to take up choline. Although colonic epithelial cells store ACh, the amount is approximately three orders of magnitude less than that in neuronal tissues, rat brain and the myenteric plexus of the distal colon of guinea-pig.
ACh release from non-neuronal epithelial cells was reported for the first time in human placenta. Interestingly, the amount of ACh released from human placenta is comparable to that observed during propionate-induced ACh release from rat colonic epithelial cells. However, ACh release occurred spontaneously in the placenta epithelial cells without any stimulation, which was not the case in colonic epithelial cells. To address the physiological role of non-neuronal ACh release from epithelium, it is important to understand whether ACh is released into the luminal or serosal side. However, previous studies have not revealed the direction of ACh release from epithelial cells of the placenta or bladder. Our study clearly demonstrated that the ACh release coupled with luminal propionate stimulation occurred on the serosal side, but not the luminal side in the rat colon.
Non-neuronal ACh is expected to act as a local signalling or trophic molecule (Klapproth et al. 1997). Our study demonstrated that cholinergic colonic ion transport is regulated not only by neuronal but also by non-neuronal mechanisms. Non-neuronally released ACh in response to luminal stimulation acts as local signalling for the muscarinic receptors of neighbouring epithelial cells following chloride secretion as well as mucin secretion (Fig. 1B). However, many questions arise, such as what kind of cells store ACh, how is the chemo-signal received, how is ACh released from the stored cells, and what other physiological roles does non-neuronally released ACh play in addition to the secretory response? We expect future studies to answer these questions.
References
Harrington AM, Hutson JM & Southwell BR (2010). Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Prog Histochem Cytochem 44, 173–202.
Hubel KA & Russ L (1993). Mechanisms of the secretory response to luminal propionate in rat descending colon in vitro. J Auton Nerv Syst 43, 219–229.
Klapproth H, Reinheimer T, Metzen J, Munch M, Bittinger F, Kirkpatrick CJ, Hohle KD, Schemann M, Racke K & Wessler I (1997). Non-neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn Schmiedebergs Arch Pharmacol 355, 515–523.
Raybould HE, Cooke HJ & Christofi FL (2004). Sensory mechanisms: transmitters, modulators and reflexes. Neurogastroenterol Motil 16 (Suppl 1), 60–63.
Wessler I & Kirkpatrick CJ (2008). Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol 154, 1558–1571.
Yajima T (1988). Luminal propionate-induced secretory response in the rat distal colon in vitro. J Physiol 403, 559–575.
Yajima T, Inoue R, Matsumoto M & Yajima M (2011). Non-neuronal release of ACh plays a key role in secretory response to luminal propionate in rat colon. J Physiol 589, 953–962. http://jp.physoc.org/content/589/4/953.long