Physiology News Magazine
A portal to pain: the transient receptor potential (TRP) vanilloid 1 channel
With a role in several pathophysiological conditions and its location in sensory neurons that respond to harmful stimuli, the non-selective ion channel TRPV1 has become a target for the control of pain. It is important to determine what regulates the function of TRPV1 for the development of new pain therapies.
Features
A portal to pain: the transient receptor potential (TRP) vanilloid 1 channel
With a role in several pathophysiological conditions and its location in sensory neurons that respond to harmful stimuli, the non-selective ion channel TRPV1 has become a target for the control of pain. It is important to determine what regulates the function of TRPV1 for the development of new pain therapies.
Features
Sara Morales-Lázaro & Tamara Rosenbaum
Instituto de Fisiología Celular, Universidad Nacional Autónoma de México, México
Sidney Simon
Duke University, North Carolina, USA
https://doi.org/10.36866/pn.94.26
Pain is an unpleasant physiological and emotional response that is commonly associated with tissue damage. Although the sensation of pain may be useful in avoiding re-injury, in general, people obviously want to avoid being in pain. Therefore research based on finding new therapeutic targets for the treatment of several diseases and the pain resulting from them has led to the discovery of various molecular regulators of ion channels that are found in neurons and whose activation will result in pain (reviewed in Julius & Basbaum, 2001). Among these channels are TRPV1, a member of the transient receptor potential (TRP) superfamily of ion channels. TRPV1 is a non-selective ion channel activated or modulated by diverse noxious stimuli such as high temperatures, changes in acidity, capsaicin, the pungent compound in chili peppers, and certain molecules released during tissue damage and inflammatory processes (Rosenbaum & Simon, 2007). Due to its role in several pathophysiological conditions (see below), TRPV1 has become a target for the control of pain. This is because it is found in sensory neurons (nociceptors) that respond to noxious or harmful stimuli. Below we outline several of the recently described endogenous small molecules that have been found to regulate the function of TRPV1 and therefore regulate the physiology of nociception or the way neurons process noxious stimuli.
TRP channels are gateways to our senses
TRP channels are found in organisms ranging from worms to humans. Their discovery has revealed them to be critical in the peripheral events in sensory physiology including osmotic regulation, calcium homeostasis, pain and inflammation, photoreception, taste, and thermosensation (reviewed in Nilius & Owsianik, 2011).
There are seven known TRP subfamilies and six of them are present in humans (reviewed in Palazzo et al. 2013): TRPC (‘canonical’), TRPA (‘ankyrin’), TRPM (‘melastatin’), TRPML (‘mucolipin’), TRPP (‘polycystin’) and TRPV (‘vanilloid’). The TRPN (‘no mechanoreceptor potential C’) channels are found in Xenopus laevis, Danio rerio and Drosophila melanogaster, but not in mammals (reviewed in Palazzo et al. 2013).

All TRP channels are tetramers composed of subunits with six transmembranal domains (S1 to S6), intracellular N- and C-termini and a region between the S5 and S6 domains that gives rise to the conduction pathway or pore (Fig. 1A). TRPV1 is a non-selective cation-permeable channel that preferentially allows the entrance of Ca2+ into the cell. The currents generated by the opening of this ion channel are of an outwardly rectifying nature. That is, less current is evident at hyperpolarizing (negative) than at depolarizing (positive) voltages (Fig. 1B). TRPV1 has six ankyrin repeats in the N-terminus (green rectangles, Fig. 2) that contain sites for molecules that regulate its activity. Among them are phosphorylation sites and an ATP binding site (reviewed in Szolcsanyi & Sandor, 2012).
A recent structure for TRPV1 was solved using novel cryo-electron-microscopy and it was proposed that the gate (the region that controls the passage of ions through the ion channel when it is opened or closed) is located intracellularly (Liao et al. 2013). However, functional data previously obtained by other groups show that a residue located further up in the middle of the pore is the one that exhibits a conformational change during the opening of the pore that can allow (or hinder) the entrance of permeant ions (Salazar et al. 2009). Thus, to date, two possible gates have been proposed: one on the basis of a static structure (Liao et al. 2013) and another one on the basis of functional data (Salazar et al. 2009).
The TRP channel family contains a subpopulation, called ‘thermo-TRPs’, that respond to changes in temperature (reviewed in Tominaga, 2007). Their threshold temperatures range from 15°C (TRPA1) to 52°C (TRPV2) (Table 1). Importantly, their thermal sensitivity decreases during inflammation. For example, for TRPV1 the threshold temperature is 42°C and it can decrease to 35°C under inflammatory conditions (reviewed in Tominaga, 2007). However, TRPV1 is not the only channel to respond to compounds found in plants. TRP channels respond to a variety of spices some of which are in mustard oil, horseradish, ginger and cloves, as well as menthol and tetrahydrocannabinol (THC), the primary psychoactive constituent of the marijuana plant (Cannabis) and others (see Table 1). Thus, their activation of TRPs can cause different types of sensations such as a cooling (TRPM8 with menthol) or a burning sensation (TRPV1 with capsaicin) (reviewed in Tominaga, 2007). Moreover, some of these thermo-TRP channels (TRPV1, TRPV2, TRPV4, TRPA1 and TRPM3) respond to changes in osmolality and to stretch and shear (frictional force produced by a fluid on a surface) stress (Table 1) (reviewed in Palazzo et al. 2013).


Characteristics of TRPV1
At present, the best-studied member of the ‘thermo-TRP’ group of channels is TRPV1. In the peripheral nervous system it is expressed mainly in small unmyelinated neurons from dorsal root (located throughout the body), nodose (in the viscera), and trigeminal (on the face) ganglia. TRPV1 has been associated with several pathophysiological conditions such as the pain and inflammation generated as a result of ischaemia, angina pectoris, diabetic neuropathy, irritable bowel syndrome, and arthritis and cancer (reviewed in Szolcsanyi & Sandor, 2012) and, consequently, has become a therapeutic target for the treatment of pain. TRPV1, like many ion channels, can exist in several states: closed, open and desensitized. In the closed state no ions can flow across the channel whereas they can in its open state. For TRPV1, the desensitized state arises from the increase in intracellular calcium that produces a conformation in which no ions flow across the channel. The activation of TRPV1 by its agonists (see Fig. 1) results in membrane depolarization of the sensory neurons due to the influx of Na+ and Ca2+ into the cell giving rise to the generation of action potentials which, when they reach the cortex, are interpreted by the central nervous system as pain. In the continued presence of a TRPV1 agonist, an interesting phenomenon takes place: instead of pain being present as long as the agonist is present, what happens is that the nerve becomes less sensitive (harder to activate) as a consequence of the rise in intracellular Ca2+, which causes TRPV1 to desensitize. This process is called neuronal
‘defunctionalization’ and is one of the reasons why TRPV1 is so intensively investigated for relieving pain (reviewed in Jara-Oseguera et al. 2008). This is also the reason that creams that contain capsaicin and sold over the counter are used to help subjects that experience certain types of pain.
Endogenous molecules that positively regulate TRPV1 function
Several molecules can regulate the activity of TRPV1 (see Fig. 2). Some of these are of an exogenous nature, such as the compounds found in plants mentioned above (Table 1). Others are endogenous molecules meaning that they are produced in our bodies.
Among endogenous regulators of TRPV1 activity there are several compounds of a lipidic (fatty) nature. Two widely studied positive regulators of TRPV1 are phosphatidylinositol 4,5-bishosphate (PI(4,5)P2 or PIP2), which is localized to the cytosolic leaflet of plasma membranes and that positively regulates the activation of TRPV1 by capsaicin and diacylglycerol (DAG) (reviewed in Julius & Basbaum, 2001). Both of these molecules bind to intracellular regions of the channel (Fig. 2). DAG is produced as a result of the calcium-dependent activation of phospholipase C (PLC) that catalyses PIP2, into DAG and inositol trishosphate (IP3) (Fig. 3). Anandamide (N-arachidonoylethanolamine), an endogenous ligand of the CB1 cannabinoid receptor (reviewed in Szolcsanyi & Sandor, 2012), also activates TRPV1 by binding to the same site in the channel as capsaicin (Fig. 2). Thus, as would be expected, other N-acyl-ethanolamines (NAEs) such as N-oleoylethanolamine (18:1 NAE or OEA) are also positive effectors of TRPV1 activity (reviewed in Morales-Lazaro et al. 2013 ).
Other TRPV1 agonists include several compounds derived from arachidonic acid, which is a polyunsaturated omega-6 fatty acid. These compounds include 20-hydroxyeicosatetraenoic acid (20-HETE), and the lipoxygenase products as hydroperoxyeicosatetraenoic acids (HPETEs) and hepoxilins (HXA3, HXB3) (reviewed in Szolcsanyi & Sandor, 2012). These molecules exert their effects on TRPV1 through the activation of signalling pathways that regulate the channel’s activity rather than directly interacting with it.
One endogenous molecule that positively regulates TRPV1 is adenosine triphosphate (ATP), which is released from tissues during inflammation and/or tissue damage and that sensitizes TRPV1 (i.e. makes it easier to activate) by directly binding to TRPV1 in a region between ankyrin repeats 1–3 of the channel (Fig. 2) (reviewed in Szolcsanyi & Sandor, 2012). Other endogenous agonists include protons (H3O+), ammonia (NH3) and divalent cations. When the extracellular pH decreases to 6.4 (as may occur during ischaemia), this potentiates TRPV1’s activity by decreasing the threshold to capsaicin and temperature. In addition, pH as low as 5.4 can, by itself, evoke TRPV1 currents (reviewed in Julius & Basbaum, 2001). Interestingly, protons regulate TRPV1 activity by binding to residues localized to the third extracellular loop, to the linker between the selectivity filter and the sixth transmembrane domain (reviewed in Jara-Oseguera et al. 2008), to the pore helix and to the linker between the S3 and S4 segments (see positive regulator 8 in Fig. 2). On the other hand, NH3, a well known irritant found in household cleaners, diffuses across cell plasma membranes where it associates with a proton leading to an increase in the pH of the cytoplasm that, in turn, will activate TRPV1 on the cytoplasmic surface through a mechanism involving a histidine residue (see positive regulator 7 in Fig. 2) (reviewed in Morales-Lazaro et al. 2013).

Finally, high concentrations of divalent cations such as Mg2+ and Ca2+ can directly activate TRPV1. Mg2+ and Ca2+ mediate their effects through extracellular residues in the channel’s structure (reviewed in Morales-Lazaro et al. 2013) (Fig. 2), the same residues that interact with protons.
Negative regulators of TRPV1 activity
To date, adenosine and cholesterol are among the very few endogenous negative regulators of TRPV1. Adenosine, a purine nucleoside, has been shown to promote analgesic effects in animals through its interaction with adenosine receptors. However, adenosine can negatively regulate TRPV1 by binding to the same region where capsaicin binds (between S2 and S3, see Fig. 2), thus leading to a decrease in the efficacy of capsaicin to activate the channel (reviewed in Morales-Lazaro et al. 2013).
Another compound that has been recently shown to modulate a variety of ion channels is cholesterol (Picazo-Juarez et al. 2011). We have shown that cholesterol down-regulates the TRPV1 activity by binding to a cholesterol recognition amino acid consensus (CRAC) motif in the S5 transmembrane domain of the channel (see negative regulator 1 in Fig. 2; Picazo-Juarez et al. 2011). Other compounds of a steroidal nature, such as 17-β-oestradiol, also have been shown to regulate the sensitivity of the nerves where TRPV1 is expressed by putatively modifying the expression levels of TRPV1 (Wu et al. 2010).
Finally, although they are not strictly endogenous molecules, resolvins, which are derived from polyunsaturated fatty acids (PUFAs) that are found in certain types of foods such as walnuts, sunflower seeds, peanuts and olive oil, have been shown to inhibit TRPV1. Resolvin E1 (RvE1) and resolvin D2 (RvD2) inhibit TRPV1 currents through a mechanism mediated by the activation of G-protein-coupled receptors (Park et al. 2011). In mice these resolvins reduce the pain associated to the activation of TRPV1 (Park et al. 2011) and thus have the potential to become useful analgesic drugs.
Molecules that activate G-protein coupled receptors also activate TRPV1
As mentioned above, some molecules are capable of regulating the activity of TRPV1 indirectly (without binding to a region in its structure). One of the molecules known to indirectly regulate TRPV1’s activity is bradykinin (BK), a nine amino acid peptide generated in inflammatory conditions. The activation of GPCRs by bradykinin results in the production of DAG, which in turn activates protein kinase C (PKC), which sensitizes TRPV1 (reviewed in Nilius & Owsianik, 2011) leading to an increase in intracellular calcium and resulting in the generation of pain and in the release of pro-inflammatory molecules (Fig. 3). In this manner, such nociceptors display an afferent response via the generation of action potentials that will ultimately lead to pain and an efferent response that will cause an inflammatory response.
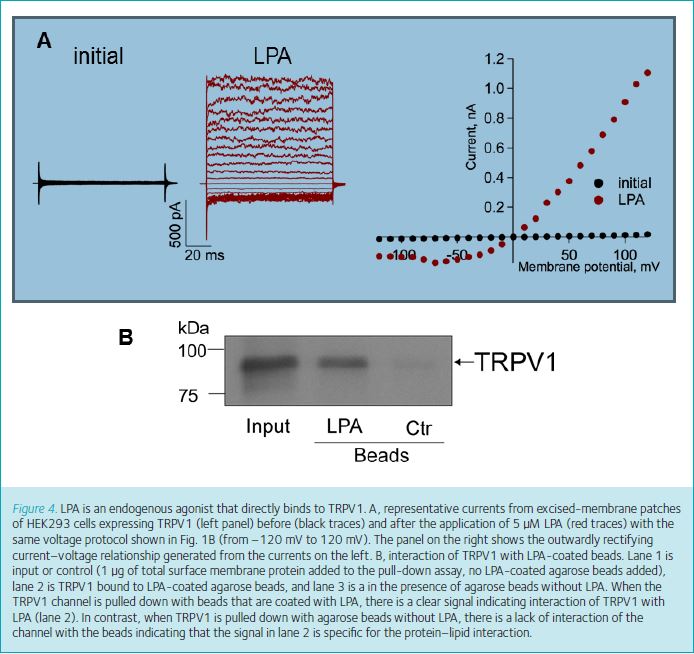
Additionally, it was recently found that lysophosphatidic acid (LPA), another GPCR agonist, also directly activates TRPV1. This lysophospholipid is involved in several important cellular processes such as neurite retraction, apoptosis and calcium mobilization (reviewed in Ueda et al. 2013). Previously, LPA’s actions were thought to be solely mediated by its interaction with specific G-protein-coupled receptors (LPA1–6) to produce neuropathic pain (reviewed in Ueda et al. 2013).
However, our group recently showed that LPA can produce acute pain by a novel mechanism which involves its direct interaction with TRPV1 (Nieto-Posadas et al. 2012). We found that the injection of LPA into the paws of mice produced an increase in the time the animals spent licking their paws as compared to the time spent licking after saline injection. The difference in these times is used as a measure of the pain the animal experiences. We demonstrated using electrophysiological and biochemical measurements that LPA directly activates TRPV1 and that this behaviour does not arise from the activation of the LPA receptors (Fig. 4). Moreover, we found that it binds to the proximal region of the C-terminus of the channel where it competes with PIP2 for a binding site (see positive regulator 5 in Fig. 2).
In summary, several physical and chemical stimuli have been shown to modulate the activity of TRPV1. This is a new and exciting area involving the regulation of pain and we are awaiting more and safer molecules to be discovered.
References
Jara-Oseguera A, Simon SA & Rosenbaum T (2008). TRPV1: on the road to pain relief. Curr Mol Pharmacol 1, 255–269.
Julius D & Basbaum AI (2001). Molecular mechanisms of nociception. Nature 413, 203–210.
Liao M, Cao E, Julius D & Cheng Y (2013). Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112.
Morales-Lazaro SL, Simon SA & Rosenbaum T (2013). The role of endogenous molecules in modulating pain through transient receptor potential vanilloid 1 (TRPV1). J Physiol 591, 3109–3121.
Nieto-Posadas A, Picazo-Juarez G, Llorente I, Jara-Oseguera A, Morales-Lazaro S, Escalante-Alcalde D, Islas LD & Rosenbaum T (2012). Lysophosphatidic acid directly activates TRPV1 through a C-terminal binding site. Nat Chem Biol 8, 78–85.
Nilius B & Owsianik G (2011). The transient receptor potential family of ion channels. Genome Biol 12, 218.
Palazzo E, Rossi F, De Novellis V & Maione S (2013). Endogenous modulators of TRP channels. Curr Top Med Chem 13, 398–407.
Park CK, Xu ZZ, Liu T, Lu N, Serhan CN & Ji RR (2011). Resolvin D2 is a potent endogenous inhibitor for transient receptor potential subtype V1/A1, inflammatory pain, and spinal cord synaptic plasticity in mice: distinct roles of resolvin D1, D2, and E1. J Neurosci 31, 18433–18438.
Picazo-Juarez G, Romero-Suarez S, Nieto-Posadas A, Llorente I, Jara-Oseguera A, Briggs M, Mcintosh TJ, Simon SA, Ladron-De-Guevara E, Islas LD & Rosenbaum T (2011). Identification of a binding motif in the S5 helix that confers cholesterol sensitivity to the TRPV1 ion channel. J Biol Chem 286, 24966–24976.
Rosenbaum T & Simon SA (2007). TRPV1 receptors and signal transduction. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, ed. Liedtke WB & Heller S. CRC Press, Boca Raton, FL.
Salazar H, Jara-Oseguera A, Hernandez-Garcia E, llorente I, Arias Olguin II, Soriano-Garcia M, Islas LD & Rosenbaum T (2009). Structural determinants of gating in the TRPV1 channel. Nat Struct Mol Biol 16, 704–710.
Szolcsanyi J & Sandor Z (2012). Multisteric TRPV1 nocisensor: a target for analgesics. Trends Pharmacol Sci 33, 646–655.
Tominaga M (2007). The role of TRP channels in thermosensation. In TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, ed. Liedtke WB & Heller S. CRC Press, Boca Raton, FL.
Ueda H, Matsunaga H, Olaposi OI & Nagai J (2013). Lysophosphatidic acid: Chemical signature of neuropathic pain. Biochim Biophys Acta 1831, 61–73.
Wu YW, Bi YP, Kou XX, Xu W, Ma LQ, Wang KW, Gan YH & Ma XC (2010). 17-β-Estradiol enhanced allodynia of inflammatory temporomandibular joint through upregulation of hippocampal TRPV1 in ovariectomized rats. J Neurosci 30, 8710–8719.