
Physiology News Magazine
A tale of three cities: the discovery of sodium/calcium exchange and some of its cardiovascular implications
How squid axons, frog muscles and the sodium/calcium exchanger built the foundation of a lifelong friendship and research collaboration.
Features
A tale of three cities: the discovery of sodium/calcium exchange and some of its cardiovascular implications
How squid axons, frog muscles and the sodium/calcium exchanger built the foundation of a lifelong friendship and research collaboration.
Features
Mordecai Blaustein
University of Maryland, USA
Harald Reuter
Professor Emeritus of Physiology, University of Bern, Switzerland
https://doi.org/10.36866/pn.96.32
‘It was the best of times’. Reminiscences, by two of the participants, of a most fortuitous and improbable set of events that took place in the Autumn of 1966: the simultaneous and completely independent discovery of the sodium/calcium exchanger (NCX) in England and in Germany. This had a very felicitous fallout.
Plymouth, England, 1966: NCX in squid axons (MPB)
In August 1966, I completed my military service (studying lobster axons) at the Naval Medical Research Institute in Bethesda, Maryland. I was awarded an NIH Special Fellowship, and my family and I moved to Cambridge, England, where I intended to study squid axon electrophysiology with Alan Hodgkin. Upon arrival in Cambridge, I learned that Peter Baker, Alan’s young protégé, was taking a mini-sabbatical to study squid axon sodium pumps at the Laboratory of the Marine Biological Association in Plymouth. Peter, who was designated to oversee the foreign research fellows studying squid axons, wanted all of that season’s effort to focus on the sodium pump. That suited me because, while in medicaI school, I did research on red cell sodium pumps (Na,K-ATPase), on which I wrote my dissertation. Hence, after visiting Vienna for the International Biophysics Congress, I left my family in Cambridge and, in mid-September, travelled to Plymouth.
Richard (Rick) Steinhardt, Richard Keynes’s postdoctoral fellow, and I shared a lab. We agreed to determine how extracellular cations affect the sodium pump-mediated extrusion of Na+ ions from 22Na+-injected squid giant axons. After teaching us how to use the axon microinjector equipment, Keynes left to help teach a course on laboratory techniques at Homburg/Saar, Germany. Fortunately, I had prior experience dissecting squid axons in John Moore and Toshio Narahashi’s lab at Woods Hole, Massachusetts.
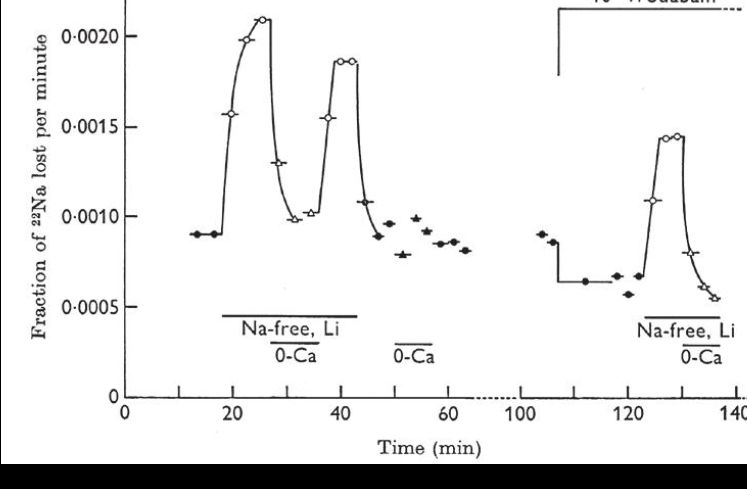
Within a week, Rick and I were obtaining reliable ouabain-sensitive (Na+ pump-mediated) Na+ efflux data. We then substituted dextrose for NaCl in the artificial sea water (ASW) bathing the axons. Unexpectedly, this induced a large, reversible Na+ efflux that was not mediated by the Na+ pump because it was not inhibited by ouabain or removal of external K+. Replacement of the NaCl by LiCl or choline Cl– gave similar results. The reversibility indicated that this was not simply a ‘leak’ of Na+ with an anion. Peter, Rick and I reasoned that the Na+ efflux involved either co-transport of Na+ with an anion, or exchange of Na+ for an external cation (we had not yet replaced the external Ca2+ or Mg2+).
The simplest test was to remove the external divalent cations. First, we replaced the Ca2+ with Mg2+. That abolished the low Na+-induced large Na+ efflux (Fig. 1) – so we had our answer: Na+/Ca2+ exchange (NCX)! To verify the result, we also replaced Mg2+ with Ca2+, but that didn’t prevent the low Na+-ASW-induced Na+ efflux. What an exciting day – and I was barely a month into my fellowship! To prove the mechanism, however, we needed to demonstrate that the Na+ efflux in low Na+-ASW was associated with a large Ca2+ influx, so we ordered 45Ca.
I was becoming exhausted by the 14–16 hour work days, but was afraid to lose momentum; I had been warned that gales were often followed by a dearth of squid. A gale did intervene, however, in early November, so I could catch my breath, and even catch up on reading. Peter had mentioned Luttgau and Niedergerke’s studies on Na+–Ca2+ antagonism in frog heart that he thought might be relevant to our work. With the lab deserted, I went down the hall to the library, and found their article (Luttgau & Niedergerke, 1958). They attributed the increased cardiac contraction in low-Na+ Ringer’s to a competition between external Na+ and Ca2+ at the cell surface. Even before finishing the article, I realized that their results might also be explained by a cardiac NCX. Here was the answer to a conundrum that puzzled me as a student and intern: how does Na+ pump inhibition by cardiotonic steroids such as digoxin and ouabain increase the force of cardiac contraction (the positive inotropic effect)? Obviously, NCX was the missing link: raising intracellular Na+ should promote NCX-mediated net gain of Ca2+, which should increase contraction efficiency (Baker et al., 1969). I was ecstatic! I treated myself to a fine dinner and excellent bottle of claret. Then, two sheets to the wind, I returned to the lab to re-read Luttgau–Niedergerke, just to be sure I wasn’t delusional. Another great day!
The 45Ca arrived at the end of the week, and Alan arrived the next Monday to see how I was getting on. At dinner, Alan listened intently as I described the data and our thoughts about their significance – but he gave no hint of whether he bought the NCX story. During the next two days, he dissected axons and modelled our data, while I set up for the Ca2+ influx experiments. Then, on Thursday, he asked if I would mind if he stayed in Plymouth for the Ca2+ influx experiments. Would I mind? I was thrilled – we’d won him over!
The next Monday, Alan and I performed the first Ca2+ influx experiment. Axon segments, tied at both ends, were incubated in 45Ca-labelled normal (Na+)-ASW or Li+-ASW. The axons were then washed and axoplasm was extruded with a miniature garden roller, weighed, dried on a planchet, and the radioactivity was counted. The first Na+-ASW axon sample gave a low count. The second was from a Li+-ASW axon, and the Panax counter nixie tubes flashed away (i.e. Ca2+ influx was much greater; Table 1). Alan broke into a broad grin; he could hardly wait to count the next samples – Na+/Ca2+ exchange was proven! When the counts were completed (long past midnight), we celebrated with ‘medicinal’ Scotch.
As Table 1 illustrates, we were very lucky. It was difficult to keep large squid alive. Therefore, while at sea, the laboratory fishermen removed the heads and placed the squid mantles in large thermoses containing iced sea water. Several hours later, we dissected the axons and started the experiments, but while the Na+ pumps were inhibited by the icy temperature, the axoplasm Na+ concentration slowly rose. This greatly augmented Na+/Ca2+ exchange compared to results from live squid axons (Table 1); we couldn’t miss the large flux differences.
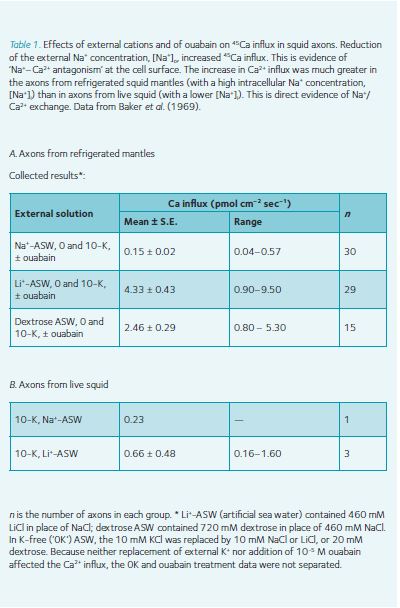
The experiments continued until Christmas (end of the squid season), and through the 1987 squid season. We recognized that a 2 Na+:1 Ca2+ exchange would not drive intracellular Ca2+ ([Ca2+]i) below 10 µM, and anticipated that [Ca2+]i was actually lower than this. The Na+:Ca2+ stoichiometry data were inconclusive, but suggested that the coupling ratio was >2 Na+:1 Ca2+ (Baker et al., 1969; Blaustein & Hodgkin, 1969).
Sadly, Peter Baker died prematurely, in 1987, just before the First International Meeting on NCX, which he planned, was convened in Stowe, England. The conference and proceedings became a memorial to Peter.
Mainz, Germany, 1966: NCX in the heart (HR)
My interest in ‘calcium and the heart’ began when I was an assistant in the Institute of Pharmacology at the University of Mainz, Germany. At that time very little was known about how Ca2+ could be involved in the beating of the heart although already in 1883 Sidney Ringer had shown that Ca2+ was essential for the beating of the frog heart (Ringer, 1883). When Silvio Weidmann, a founder of the microelectrode technique in cardiac muscle, invited me to come to the Institute of Physiology at the University of Bern, I started searching for an electrophysiologically measurable membrane current carried by Ca2+ ions in cardiac Purkinye fibres. I was lucky to discover such a current that was Ca2+ dependent and contributed to the plateau phase of the cardiac action potential and was increased by adrenaline (Reuter, 1967). This current (now called L-type Ca2+-current), its essential role in the heart beat, its molecular properties, and its regulation by neurotransmitters and drugs, became a major focus of my further scientific investigations.
If Ca2+ enters cardiac cells during excitation, a logical consequence was that some ‘Ca2+ pump’ had to extrude the ions from the cells against an electrochemical gradient. Schatzmann (1966) had shown the existence of ATP-dependent Ca2+ extrusion from human red blood cells. However, no such extrusion mechanism had so far been shown to exist in heart cells. Only several years later could Caroni & Carafoli (1981) demonstrate a Ca2+-pumping ATPase in cardiac cell membranes.
In September 1966, I attended a course on ‘Laboratory Techniques in Membrane Biophysics’ organized by Hermann Passow and Robert Stämpfli in Homburg/Saar, Germany. Under the direction of Peter Caldwell and Richard Keynes, the students had to do 22Na radiotracer flux experiments in isolated frog muscle. Those experiments showed an electroneutral Na+/Na+ exchange across the membrane that was explained by a carrier transport energetically driven by the electrochemical Na+-gradient. Hans Ussing first described this phenomenon, and called it ‘exchange diffusion’. During the membrane course Aharon Katchalsky gave a series of lectures on irreversible thermodynamics related to membrane transport. Low energy costs of Na+/Na+ exchange were mentioned by him and evoked my idea that perhaps Na+/Ca2+ instead of Na+/Na+ exchange diffusion could possibly be a transport mechanism of Ca2+ across the cardiac cell membrane. In contrast to Na+/Na+ exchange, Na+/Ca2+ exchange might provide a net transport for both ions, for example by exchanging extracellular Na+ against intracellular Ca2+.
Wilbrandt & Koller (1948) and Lüttgau & Niedergerke (1958) had already described that the concentration ratio, [Ca2+]/[Na+]2, in the Ringer solution regulated the beating strength of the frog heart. Both groups considered an antagonism between Na+ and Ca2+ as being responsible for the control of contraction. Notably, Lüttgau and Niedergerke interpreted their results by a negatively charged region on the surface of the cardiac cell where binding of Ca2+ activates contraction, while binding of Na+ inhibits it. Such Na+-Ca2+ antagonism was, however, conceptually quite different from the Na+/Ca2+-exchange I had in mind.
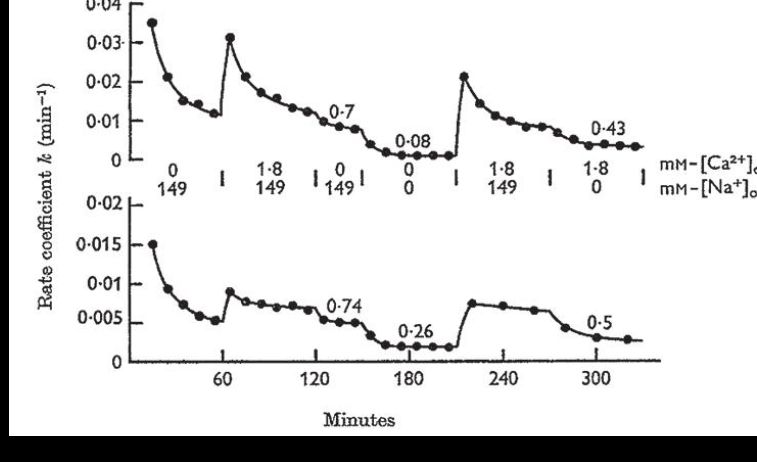
After the Homburg course I quickly started planning experiments to look for Na+/Ca2+ exchange. The technique I designed was quite simple. During a loading period with 45Ca, contractions of guinea pig auricles could be measured by a force transducer during electrical stimulation. Afterwards the isotope was unloaded by rotating the auricles through glass tubes filled with saline of different ionic composition (Fig. 2). A medical doctoral student, Norbert Seitz, helped me with the tedious rotation procedure.
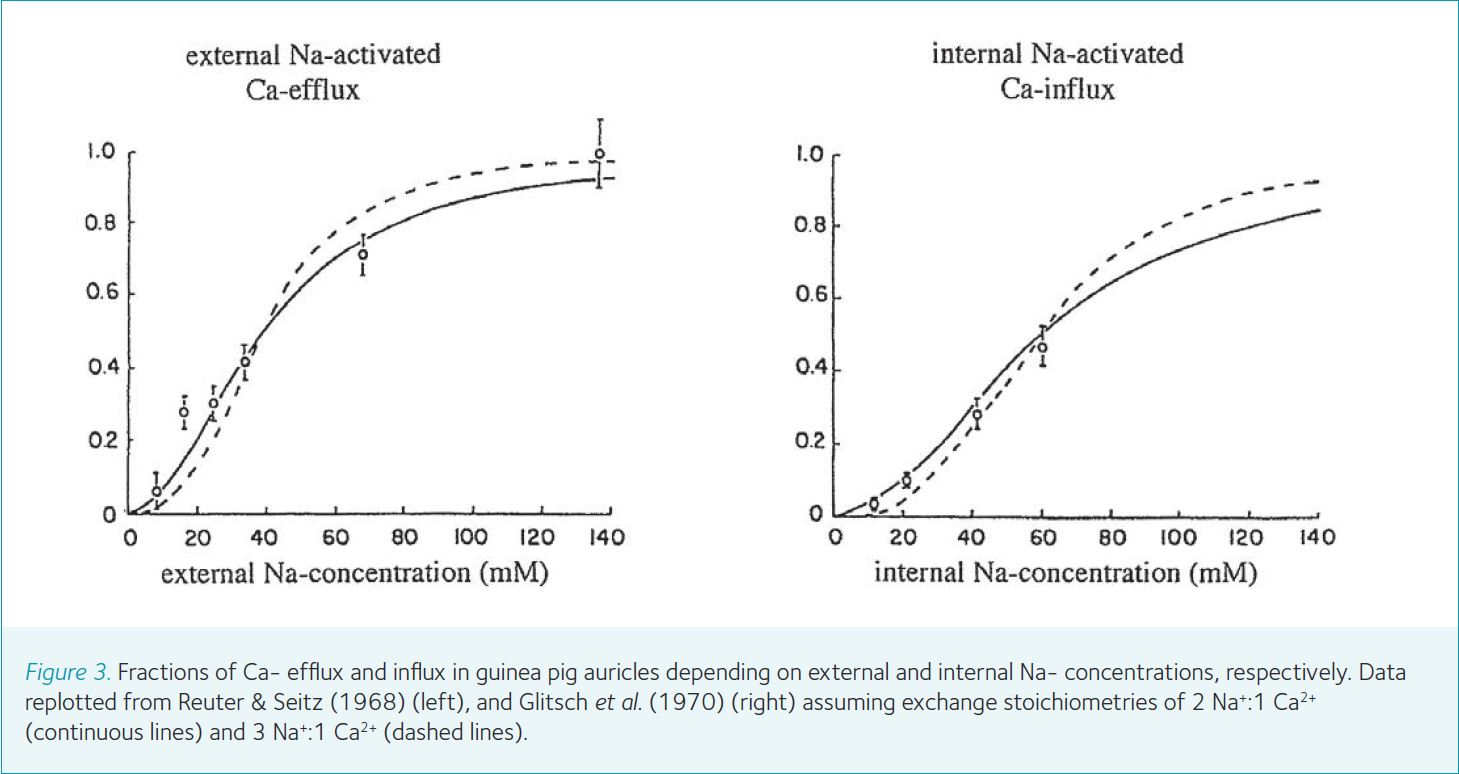
It became quickly apparent that reduction of Na+ in the external solution greatly diminished 45Ca efflux from the auricles. I was delighted that my idea of a ion exchange seemed to be correct. More quantitative 45Ca flux experiments confirmed it. Independent measurements showed that total Ca-concentration in the auricles rose when external Na+ was replaced by Li+ or sucrose and was reduced again when Na+ was readmitted. Metabolic inhibitors increased rather than decreased the rate of 45Ca efflux, in agreement with a relatively low energy demand of an exchange diffusion process, where Ca2+ extrusion from the cells depends on the Na+ and Ca2+ gradients across the cell membrane. The total 45Ca efflux could be divided into a Na+- and a Ca2+-dependent fraction. Assuming a competition of 1 Ca2+ and 2 Na+ ions for promoting Ca2+ efflux, a concentration ratio of [Ca2+]/[Na+]2 in the external medium seemed to provide a good fit to the data (Reuter & Seitz, 1968) (Fig. 3). I was misled, however, by this assumption in terms of the true stoichiometry of the exchanger.
Exchange of 2 Na+ for 1 Ca2+ is energetically insufficient to reduce the internal Ca2+ concentration, which was not known at that time, to 0.1 μM or less. Later experiments by John Reeves and Calvin Hale (1984) with cardiac membrane vesicles showed clearly a coupling ratio of 3 Na+ ions for 1 Ca2+. Thus the exchanger is electrogenic as shown by Junko Kimura and colleagues (1987). The story culminated with the cloning of the exchanger molecule by Ken Philipson (Nicoll et al., 1990).
If the driving force for net Ca2+ extrusion from the cells depends on the Na+ gradient across the cell membrane, reduction of the Na+ gradient by elevating the internal Na+ concentration, e.g. by manipulating the external K+ concentration, or by inhibiting the Na+,K+-ATPase (Na+ pump) by cardiac glycosides, should reduce Ca2+ efflux from the cells. This was, indeed, shown two years later by Glitsch, Reuter & Scholz (1970). These experiments were influenced by the squid axon studies (Baker et al., 1969). Incidentally, Hodgkin later told me, at the 1987 meeting in memory of Peter Baker, that he reviewed the Reuter & Seitz paper (1968) for The Journal of Physiology; he graciously recommended it for publication without revision. Although Rolf Niedergerke was rather skeptical, my concept of Ca2+/Na+ exchange in the heart was generally quickly accepted.
Bern, Switzerland, 1971: NCX in vascular smooth muscle (MPB & HR)
In August 1968, Blaustein accepted a faculty position at Washington University, his alma mater. He and his family moved from Cambridge to St Louis, Missouri, with a stop in Washington, DC, for the International Physiology Congress, where Reuter and Blaustein first met. This was the start of a long-lasting friendship.
In 1971 Blaustein and Reuter arranged a collaboration. The choice of venue (St Louis or Bern in late-spring and summer) was a ‘no-brainer’, so Blaustein obtained a NATO Fellowship and arranged for a mini-sabbatical, and the family headed for Switzerland. Bern also enabled them to re-connect with their Cambridge neighbours, Mani (lawyer, Bern Town Counsel and composer) and Joy Matter.
Reuter suggested that they determine whether arterial smooth muscle (ASM) has a Na+/Ca2+ exchanger – a proposal to which Blaustein readily agreed. Günther Haeusler, a vascular smooth muscle expert from Hoffmann-LaRoche, Basle, taught us how to dissect strips of rabbit thoracic aorta, in which we then measured total Ca content, 45Ca fluxes and tension. The first experiments demonstrated Na+–Ca2+ antagonism: reducing extracellular Na+ increased Ca content and enhanced contraction (Fig. 4A). We then showed that Ca2+ efflux from 45Ca-loaded aortae, but not isolated adventitia, was external Na+ dependent (Fig. 4B). Thus, ASM has a NCX mechanism that helps to regulate intracellular Ca2+ and arterial contraction (Reuter et al., 1973). We postulated that there is a close approximation between the NCX in the plasma membrane (PM) and the Ca2+ pumps in the sarcoplasmic reticulum (SR). This foreshadowed Van Breemen’s ‘buffer-barrier’ hypothesis (Chen et al., 1992) and Blaustein’s ‘PLasmERosome’ model of local Ca2+ control (Blaustein et al., 1998).
We also raised the possibility that NCX might not only help regulate vascular tone, but also contribute to the pathogenesis of hypertension. Unfortunately, the vascular smooth muscle community was reluctant to accept the Blaustein-Reuter data and ideas. The manuscript was therefore published in a non-refereed journal (Reuter et al., 1973) when Reuter was asked by Edith Bülbring to participate in a conference sponsored by the Royal Society.
Despite this skepticism, the experiments in Bern marked a turning point in Blaustein’s career, and he began to focus on Ca2+ regulation in ASM and hypertension (Blaustein, 1977). The Blaustein–Reuter ideas about the role of NCX in ASM were vindicated by immunoblot identification and immunocytochemical localization of NCX at ‘PM–SR junctions’ in ASM (Juhaszova et al., 1994). Indeed, NCX apparently plays a key role in cardiovascular physio-pathology. NCX is greatly over-expressed in cardiomyocytes during heart failure, where it promotes Ca2+ extrusion and likely contributes to left ventricular dysfunction (Studer et al., 1994; O’Rourke et al., 1999), and in arterial myocytes in many models of hypertension, where it promotes Ca2+ entry and vasoconstriction (Blaustein et al., 2012).
References
Baker PF, Blaustein MP, Hodgkin AL & Steinhardt RA (1969). The influence of calcium on sodium efflux in squid axons. J Physiol 200, 431–458.
Blaustein MP (1977). Sodium ions, calcium ions, blood pressure regulation, and hypertension: a reassessment and a hypothesis. Am J Physiol Cell Physiol 232, C165–C173.
Blaustein MP & Hodgkin AL (1969). The effect of cyanide on the efflux of calcium from squid axons. J Physiol 200, 497–527.
Blaustein MP, Juhaszova M & Golovina VA (1998). The cellular mechanism of action of cardiotonic steroids: a new hypothesis. Clin Exp Hypertens 20, 691–703.
Blaustein MP, Leenen FH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, Van Huysse JW, Zhang J & Wier WG (2012). How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol 302, H1031–1049.
Caroni P & Carafoli E (1981). The Ca2+-pumping ATPase of heart sarcolemma. Characterization, calmodulin dependence, and partial purification.
J Biol Chem 256, 3263–3270.
Chen Q, Cannell M & van Breemen C (1992). The superficial buffer barrier in vascular smooth muscle. Can J Physiol Pharmacol 70, 509–514.
Glitsch HG, Reuter H & Scholz H (1970). The effect of the internal sodium concentration on calcium fluxes in isolated guinea-pig auricles. J Physiol 209, 25–43.
Juhaszova M, Ambesi A, Lindenmayer GE, Bloch RJ & Blaustein MP (1994). Na+-Ca2+ exchanger in arteries: identification by immunoblotting and immunofluorescence microscopy. Am J Physiol Cell Physiol 266, C234–242.
Kimura J, Miyamae S & Noma A (1987). Identification of sodium-calcium exchange current in single ventricular cells of guinea-pig. J Physiol 384, 199–222.
Luttgau HC & Niedergerke R (1958). The antagonism between Ca and Na ions on the frog’s heart. J Physiol 143, 486–505.
Nicoll DA, Longoni S & Philipson KD (1990). Molecular cloning and functional expression of the cardiac sarcolemmal Na+-Ca2+ exchanger. Science 250, 562–565.
O’Rourke B, Kass DA, Tomaselli GF, Kaab S, Tunin R & Marban E (1999). Mechanisms of altered excitation-contraction coupling in canine tachycardia-induced heart failure, I: experimental studies. Circ Res 84, 562–570.
Reeves JP & Hale CC (1984). The stoichiometry of the cardiac sodium-calcium exchange system. J Biol Chem 259, 7733–7739.
Reuter H (1967). The dependence of slow inward current in Purkinje fibres on the extracellular calcium-concentration. J Physiol 192, 479–492.
Reuter H, Blaustein MP & Haeusler G (1973). Na-Ca exchange and tension development in arterial smooth muscle. Philos Trans R Soc Lond B Biol Sci 265, 87–94.
Reuter H & Seitz N (1968). The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol 195, 451–470.
Ringer S (1883). A further contribution regarding the influence of the different constituents of the blood on the contraction of the heart. J Physiol 4, 29–42.
Schatzmann HJ (1966). ATP-dependent Ca++-extrusion from human red cells. Experientia 22, 364–365.
Studer R, Reinecke H, Bilger J, Eschenhagen T, Bohm M, Hasenfuss G, Just H, Holtz J & Drexler H (1994). Gene expression of the cardiac Na+-Ca2+ exchanger in end-stage human heart failure. Circ Res 75, 443–453.
Wilbrandt W & Koller H (1948). Die Calciumwirkung am Froshherzen als Funktion des Ionengleichgewichts Zwischen Zellmembran und Umgebung. Helv Physiol Pharmacol Acta 6, 208–221.
