
Physiology News Magazine
Acidification protects skeletal muscle volume during anaerobic exercise
Biophysical measurements and theoretical modelling of resting skeletal muscle exposed to extracellular lactate demonstrate that intracellular buffering of hydrogen ions precisely offsets the effect of increased intracellular lactate on cell volume
Features
Acidification protects skeletal muscle volume during anaerobic exercise
Biophysical measurements and theoretical modelling of resting skeletal muscle exposed to extracellular lactate demonstrate that intracellular buffering of hydrogen ions precisely offsets the effect of increased intracellular lactate on cell volume
Features
Juliet A Usher-Smith, James A Fraser, & Christopher L-H Huang
Department of Physiology, Development and Neuroscience, University of Cambridge, UK
https://doi.org/10.36866/pn.65.15

Muscle exercise has long been associated with the transmembrane entry of water and an accompanying cell swelling (Kjellmer, 1964; Lannergren, 1990). Although it is now generally accepted that this water movement is the result of an increase in intracellular osmolarity (Ward et al. 1996), the precise origin and nature of this increase in osmolarity is not well understood. One candidate often implicated is the intracellular lactate produced during anaerobic metabolism. However, lactate accumulation is then accompanied by production of H+ ions from the hydrolysis of ATP and recent charge-difference modelling of skeletal muscle electrophysiology (Fraser & Huang, 2004), which suggested that steady state cell volume is determined principally by the intracellular membrane-impermeant anion content and its mean charge valency, predicted that cellular acidification would produce quantifiable reductions in cell volume through the association of H+ ions with these impermeant anions. This hypothesis was subsequently confirmed when experiments producing intracellular acidification in amphibian muscle through addition and subsequent withdrawal of NH4Cl from the extracellular solution (Fraser et al. 2005) produced predictable decreases in cell volume that were proportionate to the increase in intracellular [H+]. This suggests a mechanism by which cell volume could be conserved in the face of the production of lactate and H+ during anaerobic exercise. Thus, whilst the additional lactate would increase the intracellular osmolarity and so tend to increase cell volume, the accompanying acidification would be expected to reduce cell volume. The consequent steady-state volume would then depend on the balance between these two opposing effects.
Our study (Usher-Smith et al. 2006) establishes that, in fast-twitch amphibian muscle, these two effects precisely balance each other. By exposing resting frog muscle to extracellular solutions containing lactate we were able to reproduce the increases in intracellular lactate and H+ observed following exercise whilst avoiding the wide range of other metabolic and osmotic alterations which occur during exercise. An integration of the results from a range of experimental and theoretical techniques then enabled us to quantitatively examine the combined effects of these increases in lactate and H+ on cell volume.
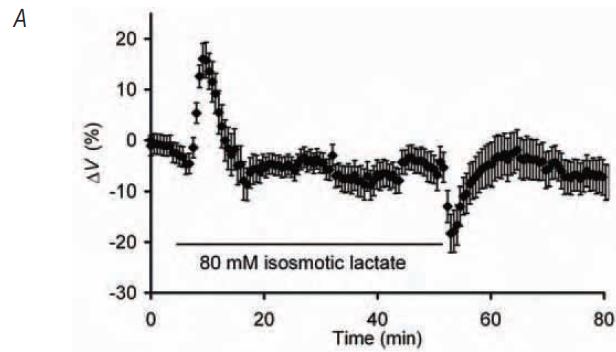
By using 1H-NMR to compare the intracellular lactate concentration, [lactate]i, resulting from exposure to extracellular lactate at a range of concentrations with that following lowfrequency electrical fatiguing stimulation, we successfully developed a protocol that increased [lactate]i in resting muscles to levels close to those following fatigue. Corresponding measurements of intracellular pH, pHi, using pH-sensitive microelectrodes similarly confirmed that the pHi in these resting muscles was within the range previously recorded in fatigued amphibian muscle. Relative cell volume was then measured using confocal xzplane scanning during and after exposure of resting fibres to lactate (Fig. 1A). Addition of extracellular lactate produced an initial increase in cell volume but this swelling was short-lived and, whilst still exposed to solutions containing lactate, the cell volume returned to its control value. Isolated increases in intracellular lactate and H+ to levels comparable with those observed following stimulation do not, therefore, significantly alter steady-state cell volume.
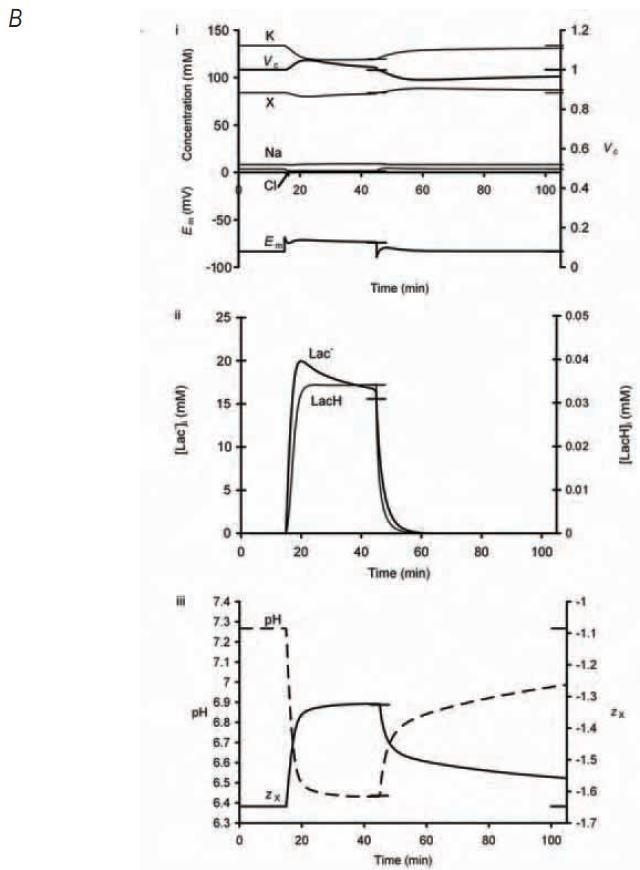
A modified charge difference model of the quantitative relationship between volume and membrane potential developed by Fraser and Huang (2004) then combined these individual cellular observations to reconstruct the osmotic and electrophysiological workings of the cellular system as a whole. In agreement with the experimental findings, simulated addition of extracellular lactate produced intracellular lactate accumulation, cellular acidification and a transient volume increase followed by recovery of the volume to initial resting values (Fig. 1B). It also clarified the mechanism responsible for these volume changes. The initial increase in cell volume could be attributed to a rapid entry of lactate and resultant increase in intracellular osmolarity but, as the pH subsequently decreases and H+ ions bind to negatively charged sites on intracellular membrane-impermeant anions, the charge on those anions, zx, becomes less negative. This results in a decrease in the net intracellular anionic charge and so allows potassium, the predominant intracellular cation, to leave the cell, reducing the intracellular osmolarity and returning the cell volume towards control values.
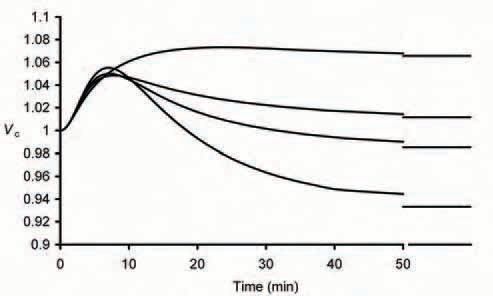
This close agreement between experimental and theoretical results additionally prompted an extension of the model to allow analysis of cellular parameters not directly amenable to experimental study but nevertheless important to the mechanisms underlying these adjustments. For example, it was possible to examine the effect of variations in total intracellular buffering capacity on the volume changes (Fig. 2). This showed that, as expected, the small initial volume increase observed on exposure to extracellular lactate is not affected but the final steady state volume is profoundly influenced by the relative intracellular buffering capacity: addition of lactate and H+ to a cell in which pHi has no influence on cell volume due to a relative buffering capacity of zero produces a 13% volume increase whilst 80 mM extracellular lactate would be expected to produce a 7% volume decrease if intracellular buffering capacity was increased by a factor of 1.5. It is only with buffering capacities similar to those actually measured in frog muscle that the final volume is not significantly different to control values as seen experimentally.
Acknowledgements
We thank our collaborators Julian Griffin and Peter Bailey (both at the University of Cambridge). This work was supported by the Medical Research Council, the Wellcome Trust, the Royal Society and Astra Zeneca with additional support from the James Baird and George Henry Lewes Funds.
References
Fraser JA & Huang CL-H (2004). A quantitative analysis of cell volume and resting potential determination and regulation in excitable cells. J Physiol 559, 459-478.
Fraser JA, Middlebrook CE, Usher-Smith JA, Schwiening CJ & Huang CL-H (2005). The effect of intracellular acidification on the relationship between cell volume and membrane potential in amphibian skeletal muscle. J Physiol 563, 745-764.
Kjellmer I (1964). The effect of exercise on the vascular bed of skeletal muscle. Acta Physiol Scand 62, 18-30.
Lannergren J (1990). Volume changes of isolated Xenopus muscle fibres associated with repeated tetanic contractions. J Physiol 420, 116P.
Usher-Smith JA, Fraser JA, Bailey PSJ, Griffin JL & Huang CL-H (2006). The influence of intracellular lactate and H+ on cell volume in amphibian skeletal muscle. J Physiol 573, 799-818.
Ward DS, Hamilton MT & Watson PD (1996). Measurement of tissue volume during non-steady state high-intensity muscle contraction. Am J Physiol 271, R1682-1690.
