
Physiology News Magazine
Adaptive responses to starvation in humans: important role for skeletal muscle PDK4
In humans, adaptations to fasting have evolved in order to survive periods of limited food resources and thus starvation. One important aspect of the adaptive response to fasting is a reduction in skeletal muscle carbohydrate utilization in order to spare glucose for those organs and tissues with an obligatory requirement for it (e.g. central nervous system). Muscle pyruvate dehydrogenase kinase (PDK4), the enzyme that impairs the rate-limiting step in glucose oxidation, is of major importance in mediating the starvation-induced shift in metabolic fuel utilization
Features
Adaptive responses to starvation in humans: important role for skeletal muscle PDK4
In humans, adaptations to fasting have evolved in order to survive periods of limited food resources and thus starvation. One important aspect of the adaptive response to fasting is a reduction in skeletal muscle carbohydrate utilization in order to spare glucose for those organs and tissues with an obligatory requirement for it (e.g. central nervous system). Muscle pyruvate dehydrogenase kinase (PDK4), the enzyme that impairs the rate-limiting step in glucose oxidation, is of major importance in mediating the starvation-induced shift in metabolic fuel utilization
Features
Kostas Tsintzas, Andrew Bennett, & Ian Macdonald
Centre for Integrated Systems Biology and Medicine, School of Biomedical Sciences, University of Nottingham Medical School, Nottingham, UK
https://doi.org/10.36866/pn.67.29

The physiological and metabolic responses to fasting have been studied extensively in many species over the last 150 years. William North published his studies on the effects of starvation and exercise on nitrogen metabolism (in which he acted as his own subject and observed an increase in whole body nitrogen excretion under both conditions) in June 1878, in the second ever issue of The Journal of Physiology (North, 1878). In the same journal Pembrey and Spriggs (1904) observed that during fasting in rats the respiratory quotient (and thus glucose oxidation) decreased quickly within the first few days of fasting and remained constant during the prolongation of fast. In 1932, Goldblatt and his co-workers in St Thomas’s Hospital, London presented evidence of carbohydrate (CHO) intolerance after starvation in healthy men (Goldblatt & Ellis, 1932). Specifically, in response to ingestion of a standardized glucose load following a 39h fast, as opposed to overnight fast, they observed an augmented blood glucose response and lower respiratory quotient and CHO oxidation rate. The starvation-induced impairment in CHO oxidation persisted even after injection of 10 units of insulin prior to glucose ingestion, prompting the authors to suggest that this may be an important adaptation which facilitates glycogen repletion in tissues (such as skeletal muscle and liver) that may have incurred a fall in glycogen content during the previous period of starvation. To our knowledge, this was the first experimental evidence of fasting-induced insulin resistance in humans.
Although these questions are still pertinent today, limited progress has been made since those pioneering studies in elucidating the precise mechanism(s) controlling the starvation-induced switch in substrate utilization and the development of insulin resistance in humans. Studies on humans from our laboratory in the 1990s using insulin clamps and stable isotopes demonstrated a reduction in whole body insulin sensitivity and a shift in basal (non-insulin) and insulin-stimulated substrate utilization from CHO to fat after 36-72h of starvation (Mansell & Macdonald, 1990; Webber et al. 1994). Importantly, starvation resulted in a marked reduction in glucose uptake by the forearm muscle both in the basal state and during insulin infusion, indicating profound muscle insulin resistance (Mansell & Macdonald, 1990).
What is the mechanism by which skeletal muscle downregulates its glucose oxidation? Some elegant animal studies performed by Mary Sugden and her coworkers in the late 1980s and early 1990s showed that suppression of skeletal muscle pyruvate dehydrogenase complex (PDC) activity plays a major role in the down-regulation of glucose oxidation in response to starvation (Sugden et al. 1993). Skeletal muscle PDC activity is inhibited by phosphorylation of the complex by pyruvate dehydrogenase kinase (PDK). The more recent identification of at least four different forms of PDK (PDK1-4) in skeletal muscle has intensified the research on their role as potential molecular regulators of glucose oxidation under a number of nutritional and pathological conditions of altered glucose homeostasis, including starvation and refeeding.
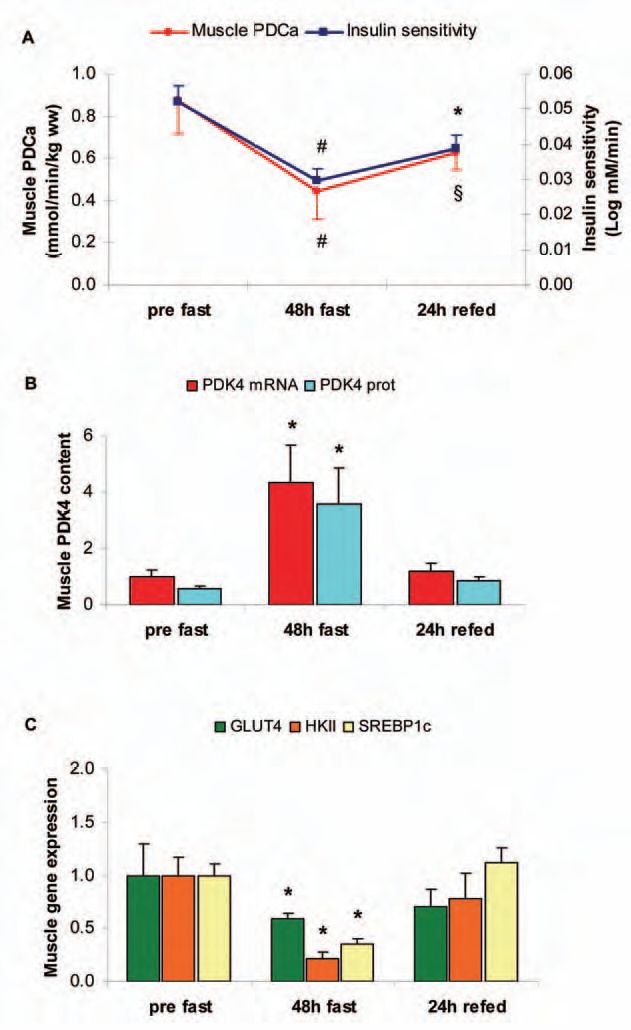
Therefore, our most recent study using insulin tolerance tests and muscle biopsies attempted to elucidate the intramuscular mechanisms underlying the adaptive response to fasting for 48h and to subsequent refeeding with a CHO-rich diet for 24h in healthy humans (Tsintzas et al. 2006). Our findings confirmed the previously demonstrated starvation-induced development of insulin resistance (as whole body insulin sensitivity decreased by ~42% after fasting) and demonstrated that this persists even after 24h of refeeding (as insulin sensitivity recovered by only half of the reduction upon refeeding) (Fig. 1A). Similarly, starvation decreased and refeeding increased skeletal muscle PDC activity, although there was a tendency for the latter to be lower after refeeding when compared with the pre-starvation value (Fig. 1A). As PDK is a major regulator of PDC, we also measured the expression of all four isoforms of PDK identified in human skeletal muscle. Starvation increased muscle PDK4 gene and protein content (without affecting the other three isoforms), whereas refeeding reversed these responses (Fig. 1B). We concluded that the selective increase in protein content of PDK4 in human skeletal muscle is an important adaptation to starvation, which most likely contributes to the long-term control of PDC activity and thus reduction of CHO oxidation under those conditions. In addition to these changes in factors affecting glucose oxidation, we also observed a decrease in the expression of key genes involved in muscle glucose uptake and phosphorylation: hexokinase II (HKII) and SREBP1c (a transcription factor that mediates the effects of insulin on HKII) were downregulated by 5- and 2.5-fold, respectively, after 48h of starvation whereas refeeding completely reversed these responses (Fig. 1C). Although muscle GLUT4 content also decreased by ~2-fold in response to starvation, its reversal was incomplete by refeeding and closely matched the slow recovery of insulin sensitivity (unpublished observation) (Fig. 1C).
What is the mechanism by which starvation increases PDK4 content in human skeletal muscle? We have recently shown for the first time in healthy humans that insulin can suppress PDK4 gene expression in human skeletal muscle (Chokkalingam et al. 2007) whereas elevated levels of plasma free fatty acids (FFA) can elevate the muscle PDK4 content (unpublished observation). Since circulating insulin concentrations decrease whereas FFA levels increase during starvation, these may be important regulators of PDK4 expression in muscle during starvation.
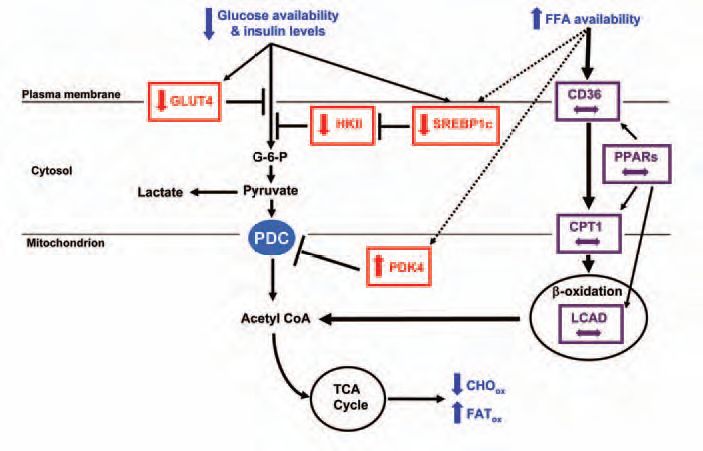
However, in contrast to what one may expect, starvation or refeeding failed to alter the content and/or activate (by phosphorylation) important muscle insulin signalling proteins (including IRS1 and 2, Akt/PKB, FOXO1) (Tsintzas et al. 2006). Our findings also showed that starvation and the concomitant increase in circulating fatty acids did not affect the expression of transcription factors [peroxisome proliferator-activated receptors (PPARs) and their coactivator PGC1α] and key genes involved in muscle fatty acid uptake and oxidation, namely fatty acid translocase (CD36), carnitine palmitoyltransferase 1 (CPT1) and long-chain acyl-CoA dehydrogenase (LCAD) (Fig. 2). Collectively, these findings suggest that, in contrast to results from animal and in vitro studies, an increase in skeletal muscle PDK4 content in fasted humans may occur in a novel manner distinct from the PPARs and insulin signalling pathways. Future studies should examine whether changes in intramuscular substrate availability/flux are responsible for the adaptive changes in glucose metabolism during fasting in humans.
In summary, it has been known for a long time that healthy humans adapt to fasting by increasing fat and reducing carbohydrate utilization in skeletal muscle in order to spare glucose for those organs and tissues (e.g. brain) with an obligatory requirement for it. We have shown for the first time that during starvation in humans, unlike rodents, regulation of fat metabolism does not require an adaptive response at the level of gene expression, implying a much greater capacity for fat oxidation than is utilized in the overnight fasted state. In contrast, changes in the content of key genes involved in glucose uptake (GLUT4), phosphorylation (SREBP1c and HKII) and oxidation (PDK4) are required to switch off glucose utilization by muscle tissue (Fig. 2). This may represent an important aspect of the molecular basis of the development of insulin resistance in metabolic conditions characterized by energy restriction.
Acknowledgments
Our work was supported by BBSRC grant 42/D1563 and a block grant from FRAME.
References
Chokkalingam K, Jewell K, Norton L, Littlewood J, vanLoon LJC, Mansell P, Macdonald IA & Tsintzas K (2007). High fat/low carbohydrate diet reduces insulin-stimulated carbohydrate oxidation but stimulates non-oxidative glucose disposal in humans: an important role for skeletal muscle PDK4. J Clin Endocrinol Metab 92, 284-292.
Goldblatt MW & Ellis RW (1932). The metabolism of carbohydrate after starvation. Biochem J 26, 991-1005.
Mansell PI & Macdonald IA (1990). The effect of starvation on insulin-induced glucose disposal and thermogenesis in humans. Metabolism 39, 502-510.
North W (1878). An Account of two Experiments illustrating the Effects of Starvation, with and without severe Labour, on the Elimination of Urea from the Body. J Physiol 1, 171-212 .
Pembrey MS & Spriggs EI (1904). The influence of fasting and feeding upon the respiratory and nitrogenous exchange. J Physiol 31, 320-345.
Sugden MC, Howard RM, Munday MR & Holness MJ (1993). Mechanisms involved in the coordinate regulation of strategic enzymes of glucose metabolism. Adv Enzyme Regul 33, 71-95.
Tsintzas K, Jewell K, Kamran M, Laithwaite D, Boonsong T, Littlewood J, Macdonald I & Bennett A (2006). Differential regulation of metabolic genes in skeletal muscle during starvation and refeeding in humans. J Physiol 575, 291-303.
Webber J, Taylor J, Greathead H, Dawson J, Buttery PJ & Macdonald IA (1994). Effects of fasting on fatty acid kinetics and on the cardiovascular, thermogenic and metabolic responses to the glucose clamp. Clin Sci (Lond) 87, 697-706.
