Physiology News Magazine
Advances in understanding new roles for astrocytes in the modulation of neuronal activity
Features
Advances in understanding new roles for astrocytes in the modulation of neuronal activity
Features
Todd A Fiacco & Cendra Agulhon
Department of Pharmacology, University of North Carolina at Chapel Hill, NC, USA
https://doi.org/10.36866/pn.72.18
The nervous system consists of two classes of cells, the neurones and glia. There are three categories of glial cells in the nervous system:
- Schwann cells and oligodendro-cytes, which produce myelin and wrap layers of myelin membrane around axons of the peripheral nervous system and the central nervous system (CNS) respectively, allowing fast electrical neuronal conduction;
- microglia, the immune cell type of the nervous system, responds to injury and disease by phago-cytosing cellular debris and triggering inflammatory responses;
- astrocytes, which ensheath synapses and blood capillaries.
Astrocytes comprise some 50% of the volume of primate brain, and have traditionally been considered to mediate metabolic, supportive and protective functions in the CNS. It is only over the past two decades that this view has changed. In this review, we discuss recent advances in the understanding of astrocyte functions in modulation of neuronal activity.
Astrocytes have emerged as a heterogeneous and multifunctional glial population. Astrocytes have been shown to coordinate the spatial positioning of oligodendrocytes during CNS development, and attract microglia and lymphocytes during inflammatory reactions, by releasing chemokines. They also maintain tight control of local ion and pH homeostasis of the interstitial space, promote neurovascular coupling, provide metabolic substrates to neurons, and synthesize glutamine from neuronally released glutamate. In addition, they play a critical role in regulation of the synaptic level of neurotransmitters, in particular glutamate, preventing excitotoxicity. Furthermore, astrocyte functions that have recently received a lot of attention involve their roles in neurogenesis, synapse formation (Ullian et al. 2004), and their ability to release neuroactive substances (called gliotransmitters), which may directly modulate neuronal excitability and transmission. In this review, we will focus on the role of astrocytes in synaptic function, and more specifically, on how astrocytes sense and react to ongoing neuronal activity and rapidly modulate this activity.

Advances in our understanding of astrocytes include new observations about their structure, organization and distribution. At the morphological level, conventional electron microscopy reveals that astrocytes are closely intertwined with synapses, suggesting an astrocytic role in synapse maintenance and function. Microinjection of single astrocytes with fluorescent dyes with the use of high resolution confocal imaging illustrates their complex morphology (Fig. 1). Individual astrocytes extend major processes, each of which ramifies into highly branched and fine processes that eventually go beyond the resolution of the light microscope. The elaborate and dense processes of each astrocyte occupy distinct anatomical domains leading to minimal overlap among adjacent astrocytes (Fig. 1) and an ordered arrangement of these cells (Nedergaard et al. 2003).


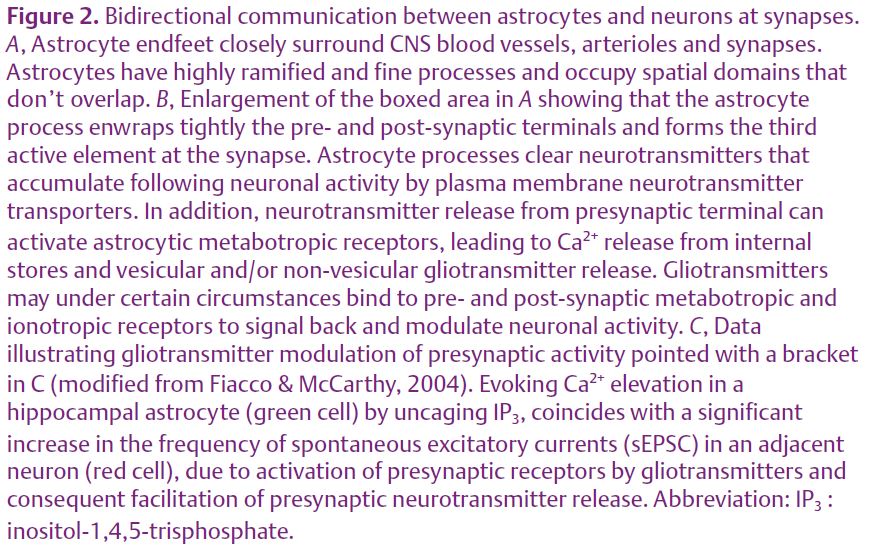
Such an arrangement allows each astrocyte to cover a distinct territory that interfaces with microvasculature and synapses (Fig. 2A). Within its own domain, each astrocyte is estimated to contact 140 000 synapses, suggesting that individual astrocytes can integrate signals from multiple synapses, and similarly signal back to multiple synapses. One remarkable consequence of this structural organization is that each synapse might be under the sole influence of a single astrocyte.
Interestingly, astrocyte processes (lamellipodia and filopodia with unique structural composition and motility) make transient highly dynamic interactions with surrounding synapses in a time scale of minutes. Thus, astrocytes are plastic and have the ability to continuously redefine their shape, possibly to match the activity level of the synapse and modulate neurotransmission.
Furthermore, astrocytes form communicating networks. Many astrocytes are indeed highly metabolically and electrically coupled by gap junctions inter-connecting adjacent astrocytic processes from the same cell and between other astrocytes. Gap junctions provide a direct path for fast exchange of ions (such as calcium) and small molecules, which represents one strategy for the networking of astrocyte populations (Scemes & Giaume, 2006). Gap junctional proteins, connexins, are targets of many endogenous active molecules indicating that astrocytic networks are subject to plasticity.
Other pivotal discoveries that have opened new perspectives for astrocyte functions include:
- astrocytes have many of the same neurotransmitter receptor signalling systems as neurons;
- astrocytes communicate with one another and with neurons through chemical signals as opposed to action potentials;
- astrocytes have the capability to release gliotransmitters, such as glutamate, ATP, and D-serine, under specific experimental conditions to modulate neuronal excitability and transmission.
Since astrocytes are electrically non-excitable, astrocytes received little attention during the past century given that the electrical language of neurons was thought to be the only way for rapid CNS communication.
Consequently, astrocytes had been presumed to be unable to initiate dialogue with neurons. However, metabotropic receptors, ion channels, and transporters allow astrocytes to receive neuronal signals. Recent advances in calcium (Ca2+) imaging methods have enabled observation of astrocyte G-protein coupled receptor (GPCR)-mediated Ca2+ elevations in response to spillover of neurotransmitter by neuronal presynaptic terminals. Depending upon the level of neuronal activity (spanning basal activity to physiological and pathological levels of excitation) astrocyte Ca2+ transients can either remain restricted to microdomains of processes, or propagate as a Ca2+ wave intracellularly or between many astrocytes (Porter et al. 1996; Nett et al. 2002; Peters et al. 2003; Wang et al. 2006). Therefore, astrocytes might form functional networks and communicate with each other through intracellular and intercellular Ca2+ waves, providing a mechanism for the propagation of information over long distances. Although it has not been addressed yet, it would be interesting to check if Ca2+ microdomains are preferen-tially involved in basal physiological states when Ca2+ waves would be associated with a high level of neuronal activity (e.g. learning processes) and pathological states (e.g. epilepsy or brain ischemia). Properties of Ca2+ oscillation dynamics generated in astrocytes, including their amplitude frequency and network activity, are governed by the intrinsic properties of astrocytes and neuronal inputs. For instance, synchrony of astrocyte Ca2+ activity has been found to be driven by underlying networks of neurons (Aguado et al. 2002; Agulhon et al. 2007).
Interestingly, it has been reported (in vitro and in acute brain slices in situ) that Ca2+ rises in astrocytes may then trigger gliotransmitter release at the astrocytic processes to signal back and modulate neuronal activity locally by regulating both pre- and post-synaptic functions (Fig. 2B,C; Parri et al. 2001; Fiacco & McCarthy, 2004). This discovery that astrocytes have the capability to be excited and send back messages to neurones has expanded our appreciation of the complexity of CNS communication to an integrated network of both neuronal and astrocytic circuitries. However, the physiological relevance of gliotransmitter release still remains to be elucidated in vivo.
Although there is a large body of evidence for gliotransmitter release from astrocytes in brain slices affecting neuronal function, the mechanisms of gliotransmitter release are still controversial, since the majority of studies on release mechanisms have been carried out in vitro. Calcium-dependent and/or Ca2+-independent effluxes through hemi-channels, P2X7 receptors, volume-regulated ion channels, and vesicular release of transmitters, have all been demonstrated (Fiacco & McCarthy, 2006). In astrocytes, Ca2+ signals generated by GPCRs are often encoded by different agonist concentrations eliciting Ca2+ elevations of variable kinetics (kinetic modulation). In addition, the Ca2+ signal can be conveyed by oscillations of intracellular Ca2+, where agonist concentration determines the frequency of Ca2+ oscillations (frequency modulation). Astrocytes might encode external neuronal inputs into Ca2+ signals and decode them into specific types of gliotransmitter secretion.
Overall, these findings have enhanced our view of glial cell function and are among the highlights of cellular neuroscience research over the last decade. The physiological consequences of astrocyte-neuron communication on the modulation of neuronal activity and synaptic transmission suggest that astrocytes may be actively involved in information processing in the CNS in vivo.
References
Aguado F, Espinosa-Parrilla JF, Carmona MA & Soriano E (2002). Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ. JNeurosci 22, 9430–9444.
Agulhon C, Platel JC, Kolomiets B, Forster V, Picaud S, Brocard J, Faure P & Brulet P (2007). Bioluminescent imaging of Ca2+ activity reveals spatiotemporal dynamics in glial networks of dark-adapted mouse retina. J Physiol 583, 945–958.
Fiacco TA & McCarthy KD (2004). Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J Neurosci 24, 722–732.
Fiacco TA & McCarthy KD (2006). Astrocyte calcium elevations: properties, propagation, and effects on brain signaling. Glia 54, 676–690.
Nedergaard M, Ransom B & Goldman SA (2003). New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26, 523–530.
Nett WJ, Oloff SH & McCarthy KD (2002). Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J Neurophysiol 87, 528–537.
Parri HR, Gould TM & Crunelli V (2001). Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci 4, 803–812.
Peters O, Schipke CG, Hashimoto Y & Kettenman H (2003). Different mechanisms promote astrocyte Ca2+ waves and spreading depression in the mouse neocortex. J Neurosc 23, 9888–9896.
Porter JT & McCarthy KD (1996). Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci 16, 5073–5081.
Scemes E & Giaume C (2006). Astrocyte calcium waves: what they are and what they do. Glia 54, 716–725.
Ullian EM, Christopherson KS & Barres BA (2004). Role for glia in synaptogenesis. Glia 47, 209–216.
Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T & Nedergaard M (2006). Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat Neurosci 9, 816–823.