
Physiology News Magazine
Analyses of motor unit firing patterns and synchrony contribute to our understanding of tremor mechanisms in Parkinson’s disease
Muscle force tremors and neurogenic components of tremors of body segments reflect rhythms in motor unit activities. The analysis of motor unit firing in Parkinson’s disease discloses the characteristics of the synchrony and discharge patterns underlying tremor, and also furnishes estimates of their impacts on the tremor amplitude. It further reveals features of the neural input provided by the tremor generator to motoneuron populations.
Features
Analyses of motor unit firing patterns and synchrony contribute to our understanding of tremor mechanisms in Parkinson’s disease
Muscle force tremors and neurogenic components of tremors of body segments reflect rhythms in motor unit activities. The analysis of motor unit firing in Parkinson’s disease discloses the characteristics of the synchrony and discharge patterns underlying tremor, and also furnishes estimates of their impacts on the tremor amplitude. It further reveals features of the neural input provided by the tremor generator to motoneuron populations.
Features
Constantinos N Christakos (1,2), Sophia Erimaki (1,2), Evangelos Anagnostou (3) and Dimitri Anastasopoulos (3)
1: Laboratory of Systems Physiology, Medical School, University of Crete, Heraklion, Greece
2: Computational Neuroscience Group, Institute of Applied and Computational Mathematics, FORTH, Heraklion, Greece
3: Laboratory of Physiology and Clinical Neurophysiology, School of Nursing, University of Athens, Greece
https://doi.org/10.36866/pn.79.28

After decades of research, the mechanisms of rest and postural tremor representing common signs of Parkinson’s disease (PD) remain uncertain. There is ample evidence implicating supraspinal centres (basal ganglia, thalamus) and strong neural synchrony in the generation of the tremor rhythm. Observations involving spinal/peripheral action in tremor genesis also exist. An overview of such findings has been presented by Christakos et al. (2009).
Tremor investigations in PD have so far focused on cerebral activities, yet the neurogenic components of rest and postural tremor result from muscle force tremors caused by rhythmical motor unit (MU) activities. Furthermore, MU populations represent sites of convergence of various central and peripheral neural influences. Thus, the firing synchrony and patterns of MUs, apart from being responsible for the tremor formation and properties, also carry information on the neural input provided by the tremor generator to motoneuron (MN) populations.
In a recent study (Christakos et al. 2009), we recorded force tremor and simultaneous MU spikes from a finger muscle contracting against a force transducer in 19 parkinsonian subjects and 19 age-matched control subjects.
We analysed the MU firing synchrony using a combination of coherence and cross-correlation computations on MU–tremor pairs (Christakos, 1997). This technique, being experimentally and computationally efficient, is particularly suitable in cases of patients with movement disorders. We also studied the MU firing patterns using higher-order interspike interval (ISI) analysis, which is an appropriate technique in cases of complex rhythmical firing. We then considered the observed characteristics of MU firing in relation to both the tremor properties and the features of the neural input to the MUs.
Rhythmical firing synchrony within the active motor unit population
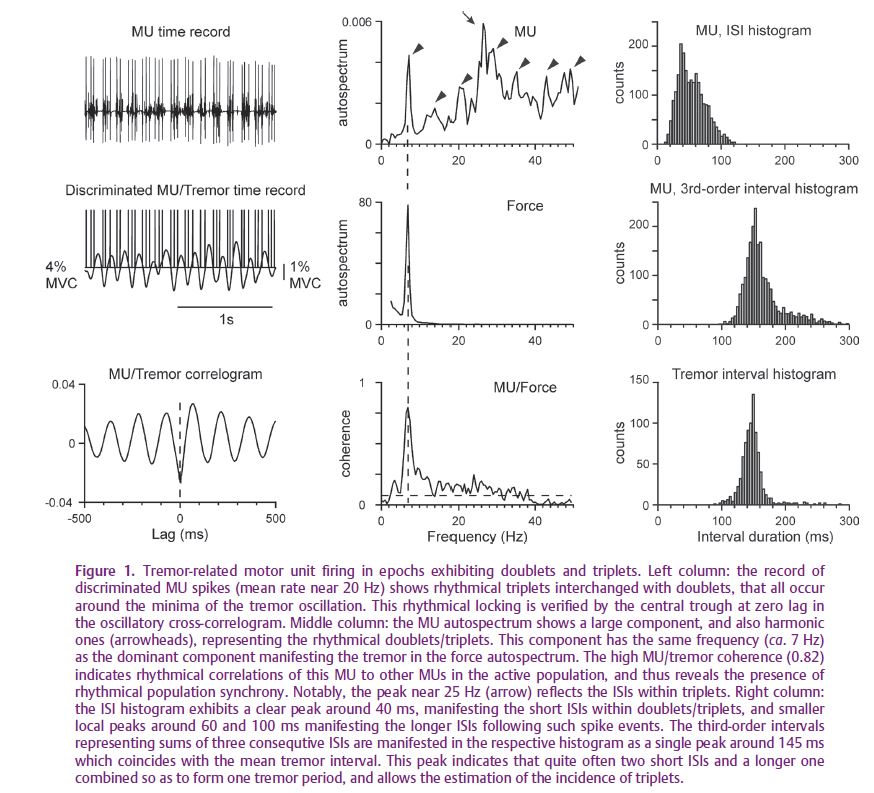
In the two minute records from our patients there were epochs (Fig. 1) where MUs exhibited rhythmical spike doublets and triplets at the tremor frequency (observed range 4.5–8.0 Hz). The mean ISIs within such spike-events were of the order of 50 ms. These epochs were randomly interchanged with epochs showing the normal rhythmical firing at the MUs’ individual discharge rates. Spike doublets were first studied by Dietz et al. (1974) and Dengler et al. (1989) who also described an association with overt tremor.
In all epochs of the patients’ records, all 50 studied MUs, irrespective of their discharge rates, exhibited a firing component that was coherent to the tremor (Fig. 1). Moreover, the synchronous MU components were in-phase, according to the MU–tremor cross-correlograms which indicated a clear tendency for MU spikes to occur around the tremor minima. A widespread and in-phase tremor-related synchrony was also present in the control subjects, as it is in younger normal adults (Christakos et al. 2006).
The strength of the tremor-related synchrony ranged from very small to very large in the patients’ contractions, as it did in those of the control subjects. In terms of MU–tremor coherence, the observed range in both groups was 0.10 to 0.90, where 1.0 is the maximal coherence value for perfect linear correlation. However, this coherence was on average significantly higher in the patients’ group (0.50 vs. 0.36).
Firing patterns of motor units
The other clear difference to the control group was that the synchronous MU components partly represented rhythmical sequences of spike events (doublets/triplets). This behaviour has important consequences for parkinsonian tremor.
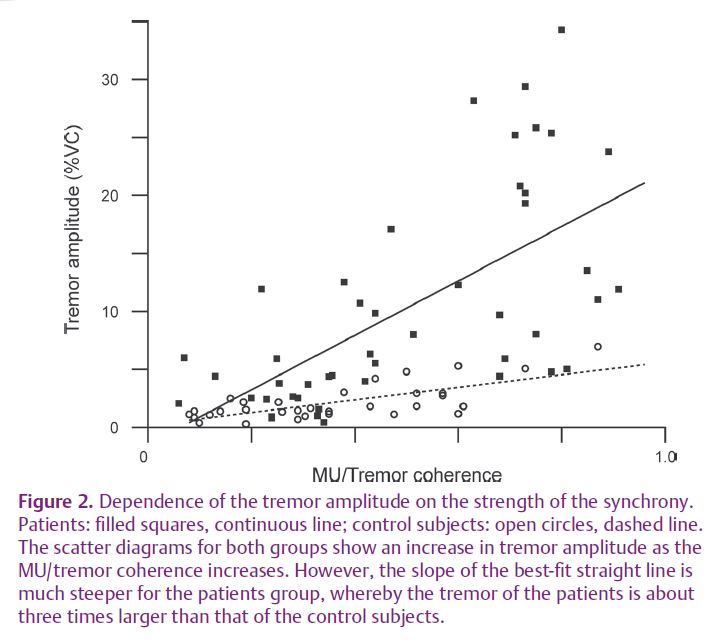
Specifically, the tremor amplitude in the 2 minute records of the patients was much larger than that in the control subjects. As seen in Fig. 2, both amplitudes increased with increasing MU–tremor coherence, but, throughout, their ratio was around 3.0. Further, the observed coherence difference between the two groups (0.14) could only account in small part for this ratio. Therefore, the large tremor of the patients is primarily due to the rhythmical spike doublets and triplets.
The study of this phenomenon therefore presents particular interest. Higher-order ISI analysis (Fig. 1) enabled us to quantify the incidences of doublets/triplets and to arrive at the following simple rule: for MUs firing above the tremor frequency, all spikes in excess participate in the formation of doublets; there are thus doublets in all tremor cycles when the MU rate is twice the tremor frequency; similarly, for MUs firing at even higher rates, up to three times the tremor frequency, there are triplets interchanged with doublets.
In both the patients and the control subjects, no MU was found to fire below the tremor frequency, i.e. the MU recruitment rate coincided with the latter frequency (see also Erimaki & Christakos, 2008). Given the size principle governing the recruitment and rate coding of MUs, the doublets/triplets were therefore exhibited by medium-sized and small MUs. The numerous such MUs contribute rhythmical sequences of twitch doublets/triplets, i.e. large force pulses, to the tremor, thus causing its enhancement.
Neural input to motoneuron populations
The observed characteristics of the tremor-related synchrony (widespread, in-phase) are consistent with the concept of a common rhythmical input provided by the tremor generator to the MN population. According to the observed rule regarding the incidences of doublets/triplets, MUs firing at high mean rates exhibit, in response to this synaptic input, a slower imposed rhythm of spike events.
As we previously discussed (Christakos et al. 2009), the other observed characteristic, namely, the fairly fixed (ca. 50 ms) mean ISIs within doublets/triplets, probably manifests features of this rhythmical synaptic input. Accordingly, the tremor-related input may exhibit multiple bursts per cycle, separated by ca. 50 ms; alternatively, it may have an additional 20 Hz component, such as the 11–30 Hz components that exist in movement-related neural activities. These possibilities could therefore facilitate the exploration of the neural generator of parkinsonian force tremor.
Postural tremor which involves voluntary muscle contractions, but also rest tremor, is known to be largely neurogenic. The observed MU behaviours are therefore relevant for such parkinsonian tremors. More generally, analyses of MU firing synchrony and patterns could facilitate the study of tremors in other movement disorders.
Acknowledgements
This study was supported by the European Social Fund and National Resources Grant PYTHAGORAS-II 2090 and the ELKE of the University of Athens.
References
Christakos CN (1997). On the detection and measurement of synchrony in neural populations by coherence analysis. J Neurophysiol 78, 3453–3459.
Christakos CN, Erimaki, S, Anagnostou E & Anastasopoulos D (2009). Tremor-related motor unit firing in Parkinson’s disease: implications for tremor genesis. J Physiol 587, 4811–4827. http://jp.physoc.org/content/587/20/4811.long
Christakos CN, Papadimitriou NA & Erimaki S (2006). Parallel neuronal mechanisms underlying physiological force tremor in steady muscle contractions of humans. J Neurophysiol 95, 53–66.
Dengler R, Gillespie J, Argenta M, Elek J, Wolf W & Struppler A (1989). The impact of paired motor unit discharges on tremor. Electromyogr Clin Neurophysiol 29, 113–117.
Dietz V, Hillesheimer W & Freund HJ (1974). Correlation between tremor, voluntary contraction, and firing pattern of motor units in Parkinson‘s disease. J Neurol Neurosurg Psychiatry 37, 927–937.
Erimaki S & Christakos CN (2008). Coherent motor unit rhythms in the 6–10 Hz range during time-varying voluntary muscle contractions: neural mechanism and relation to rhythmical motor control. J Neurophysiol 99, 473–483.
