Physiology News Magazine
Angiotensin II and intestinal glucose uptake
An enterocyte renin-angiotensin system has been shown to be involved in AT1 receptor mediated sodium-dependent glucose transport and thus intestinal glucose uptake. How are these findings clinically relevant to intestinal glucose transport in diabetic conditions?
Features
Angiotensin II and intestinal glucose uptake
An enterocyte renin-angiotensin system has been shown to be involved in AT1 receptor mediated sodium-dependent glucose transport and thus intestinal glucose uptake. How are these findings clinically relevant to intestinal glucose transport in diabetic conditions?
Features
Po Sing Leung
Department of Physiology, Faculty of Medicine, The Chinese University of Hong Kong, Shatin, New Territories, Hong Kong, China
https://doi.org/10.36866/pn.72.21

The circulating renin-angiotensin system (RAS) has a major endocrine role in the regulation of arterial blood pressure, electrolyte and fluid homeostasis by acting on vascular smooth muscle, adrenal aldosterone secretion and renal electrolyte reabsorption. Dysregulation of the RAS thus leads to renal, cardiovascular and metabolic diseases. The classic RAS consists of several key elements: a liver-derived precursor angiotensinogen and two critical enzymes for the system, namely kidney-derived renin and lung-bound angiotensin-converting enzyme (ACE). The sequential actions of these two enzymes on angiotensinogen produce plasma angiotensin I and angiotensin II, respectively. Most major actions, if not all, of the RAS are mediated by the peptide angiotensin II, the physiologically active component of the system. The effects of angiotensin II are, in turn, mediated by two angiotensin II receptor subtypes, namely AT1 receptors and AT2 receptors (De Gasparo et al. 2000).
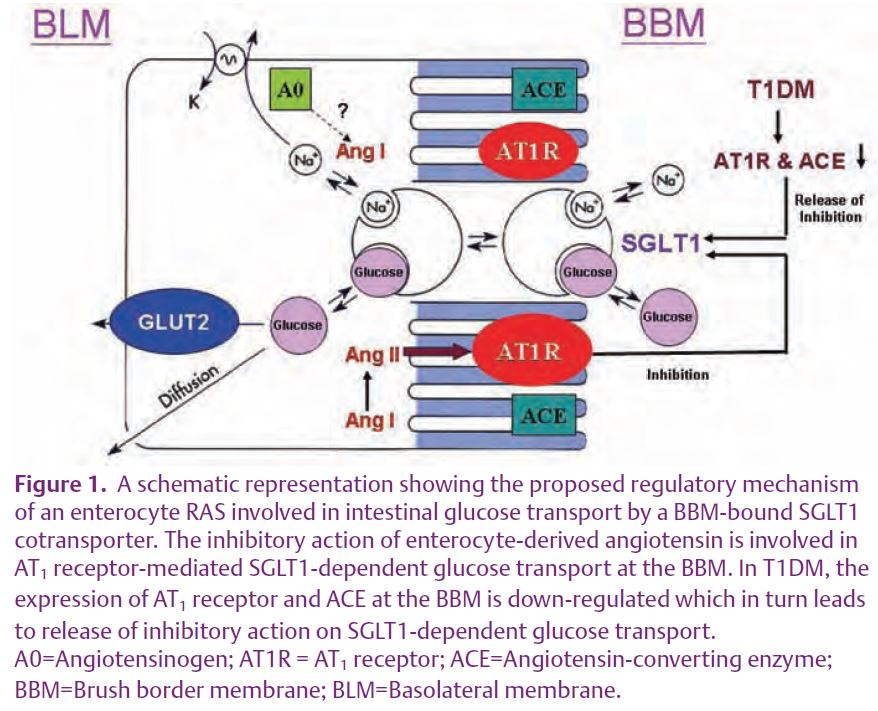
In addition to this hormonal or circulating RAS, the past two decades has seen a recognition of functional RAS that exert autocrine, paracrine and/or intracrine control of local intercellular and intracellular functions (Paul et al. 2006). These functions include, to name a few, cell proliferation, apoptosis, free radical generation, hormonal secretion, local blood flow, proinflammatory and profibrogenic actions. The recent identification of a functional RAS in the pancreas and the liver has focused interest on the gastrointestinal tract as a target of RAS action (Leung, 2007; Leung et al. 2003). Evidence for the existence of a functional RAS in the intestine has been previously reported (Paul et al. 2006). However, there are no solid data for the involvement of enterocyte-derived angiotensin II in the local control of intestinal transport. Two primary functions of the intestine are the digestion of food and absorption of electrolytes and nutrients such as glucose. The absorption of glucose involves an entry-and-exit mechanism mediated by two separate protein carriers located in the brush border membrane (BBM) and basolateral membrane (BLM) of enterocytes (Wright et al. 1994). This established model is energized by a BBM bound ATP-dependent Na+ pump (SGLT1) that maintains a sodium gradient favoring the entry of Na+ with the concomitant cotransport of glucose or galactose into the enterocytes. A second carrier (GLUT2) located at BLM then facilitates the exit of glucose from the cell (Fig. 1). Notwithstanding the involvement of this secondary active transport, the regulation of SGLT1, and consequently of glucose uptake at the BBM of enterocytes is yet to be elucidated.
We have recently found compelling evidence for the existence of an enterocyte RAS that generates angiotensin II locally and leads to a rapid inhibition of SGLT1-mediated intestinal glucose uptake (Wong et al. 2007). In this study, expression of key RAS components at the gene and protein levels were examined in jejunal and ileal enterocytes. Mucosal uptake of glucose by everted intestinal sleeves before and after addition of angiotensin II to the mucosal buffer was measured in the presence or absence of losartan, an AT1 receptor antagonist. Results revealed that enterocytes express angiotensinogen, ACE, and AT1 and AT2 receptors; AT1 receptor and angiotensinogen proteins were specifically localized to the BBM.
Expression of angiotensinogen (a mandatory component of a local RAS) and AT1 and AT2 receptors, but not ACE, was greater in the ileum than the jejunum. Addition of angiotensin II to mucosal buffer inhibited phlorizin-sensitive (SGLT1-dependent) jejunal glucose uptake in a rapid and dose-dependent manner and reduced the expression of SGLT1 at the BBM. Losartan attenuated the inhibitory action of angiotensin II on glucose uptake. Despite this inhibitory effect on glucose uptake, angiotensin II did not affect jejunal uptake of L-leucine, suggesting a specific action on glucose (Wong et al. 2007). Figure 1 summarizes the involvement of an enterocyte RAS which acts as an autocrine control in the rapid inhibition of SGLT1-dependent glucose uptake.
In keeping with these findings, our next question to be addressed is this: what is the clinical relevance of this enterocyte RAS in the AT1 receptor-mediated SGLT1-dependent glucose uptake in the intestine? We hypothesized that the expression of the RAS components at the enterocyte BBM is subject to regulation by prevailing physiological and pathophysiological conditions in the jejunum and ileum.
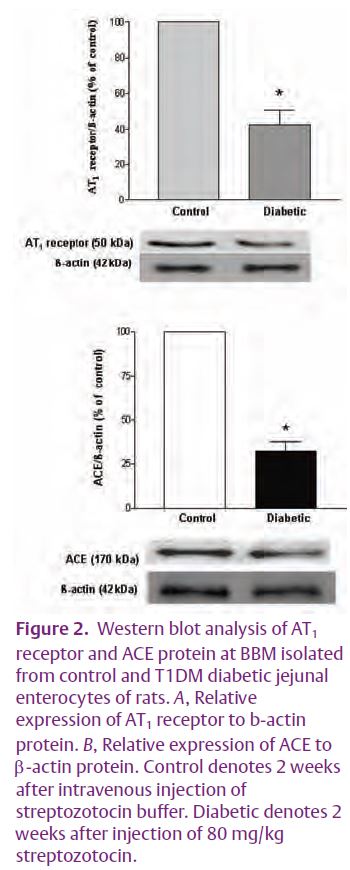
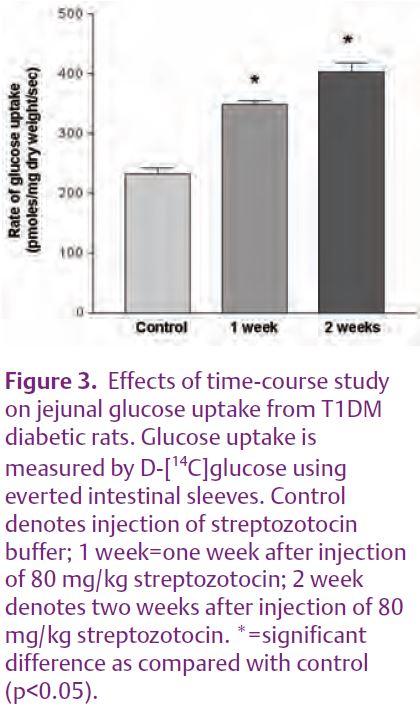
We tested this hypothesis by examining the expression of enterocyte RAS components in hypoxia and in type 1 diabetes mellitus (T1DM). Preliminary results showed that the expression of RAS components was down-regulated by hypoxia and even more so by streptozotocin-induced T1DM. The expression of the AT1 receptor and of ACE at the enterocyte BBM was significantly decreased in T1DM (Fig. 2). In addition, the intestinal uptake of D-[14C] glucose was stimulated in a time-course dependent manner in the mucosa of the everted intestinal sleeve (Fig. 3). Consistent with our results, enhanced glucose uptake across kidney proximal tubule BBM due to a reduced expression of angiotensin II receptors, has been previously observed in streptozotocin-induced T1DM (Cheng et al. 1994). Interestingly, glucose uptake across the kidney proximal tubule BBM is strikingly similar to that seen in enterocyte BBM. Taken together, these data prompt us to speculate that down-regulation of the enterocyte RAS during T1DM may be responsible for the enhanced SGLT1-mediated glucose uptake, a finding that is closely relevant to patients with T1DM and/or T2DM (see Fig. 1).
Given this evidence for the involvement of enterocyte RAS in diabetes, it is important that we now elucidate the precise mechanisms by which enterocyte-derived angiotensin II, via mediation of the AT1 receptor, exerts its effect on SGLT1-dependent intestinal glucose transport.
References
Cheng HF, Burns KD & Harris RC (1994). Reduced proximal tubule angiotensin II receptor expression in streptozotocin-induced diabetes mellitus. Kidney Int 46, 1603–1610.
De Gasparo M, Catt KJ, Inagami T, Wright JW & Unger T (2000). International Union of Pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52, 415–472.
Leung PS (2007). The physiology of a local renin-angiotensin system in the pancreas. J Physiol 580, 31–37.
Leung PS, Suen PM, Ip SP, Yip CK, Chen G & Lai BS (2003). Expression and localization of AT1 receptors in hepatic Kupffer cells: its potential role in regulating a fibrogenic response. Regul Pept 116, 61–69.
Paul M, Poyan MA & Kreutz R (2006). Physiology of local renin-angiotensin systems. Physiol Rev 86, 747–803.
Wong TP, Debnam ES & Leung PS (2007). Involvement of an enterocyte renin-angiotensin system in the local control of SGLT1-dependent glucose uptake across the rat small intestinal brush border membrane. J Physiol 584, 613–623.
Wright EM, Hirayama BA, Loo DDF, Turk E & Hager K (1994). Intestinal sugar transport, pp. 1751-1772. In Johnson LR (ed) Physiology of the gastrointestinal tract, 3rd ed. New York, Raven Press.