Physiology News Magazine
Are you ruled by your head or your heart?
Neurogenic versus myogenic debate on the origin of the heartbeat
Features
Are you ruled by your head or your heart?
Neurogenic versus myogenic debate on the origin of the heartbeat
Features
Mark Boyett, University of Copenhagen, Denmark
Alicia D’Souza, University of Manchester, UK
https://doi.org/10.36866/pn.120.18
“One of the most remarkable phenomena of nature is the incessant rhythmic action of the heart” (Fye, 1987). For around 1,000 years, there was a debate about whether the heartbeat is neurogenic or myogenic in origin, i.e. whether the contraction of the heart is initiated by motor nerve activity (like that of skeletal muscle) or whether the contraction is initiated within the muscle of the heart itself. The original debate only reached a conclusion in the 19th century, but there is now a new, related neurogenic versus myogenic debate.
The original neurogenic versus myogenic debate on the origin of the heartbeat
Galen was a physician, surgeon and philosopher in the Roman Empire and, when he observed that the heart continued to beat after it had been excised, he concluded that, “The pulsative faculty of the heart has its source in its own substance” (Fye, 1987). This is the essence of the myogenic theory of the origin of the heartbeat and can be considered the original thesis. In the 19th century, the French physiologist and physician César Julien Jean Legallois observed that the mammalian heart stopped beating when the spinal cord was abruptly crushed and he concluded that the heartbeat is neurogenic in origin (the antithesis) (Legallois, 1812); Fig.1A shows the title page of the English translation of Legallois’s publication. As the understanding of the autonomic nervous system grew and the intracardiac ganglia were discovered, ideas evolved and the German physiologists Eduard and Ernst Weber concluded that the sympathetic nerves acting via the intracardiac ganglia were the motor nerves of the heart – the proposed involvement of the ganglia could explain why the isolated heart carries on beating after the autonomic nerves innervating the heart are sectioned (Fye, 1987).

However, Walter Holbrook Gaskell, working in Trinity College Cambridge, went on to disprove the neurogenic theory and prove the myogenic theory of the heartbeat. In 1880 he wrote that most physiologists attributed the heartbeat to “the action of certain ganglion cells situated in the heart itself, while the cardiac muscular tissue is credited with the purely subordinate role of responding to the impulses generated in these nerve cells” (Gaskell, 1880). Gaskell’s central observation was that the apex of the tortoise heart, although devoid of ganglion cells, has the ability to beat rhythmically like other parts of the heart (Gaskell, 1883). Furthermore, he showed that the conduction of the impulse through the muscle of the heart “does not depend upon the nerve trunks between sinus and ventricle.” He showed that, “if the auricular muscle be carefully separated from the ventricle and along each side of the inter- auricular basal wall, so that the ventricle is connected with the sinus and auricle only by the band of tissue which contains the large nerves and coronary veins, then the sequence between auricle and ventricle is entirely lost” (Gaskell, 1882). Instead he showed that the impulse is able to conduct through the muscle of the heart (Gaskell, 1882).
Today we know that cardiomyocytes are electrically and mechanically coupled together, allowing the impulse or action potential to propagate from cell to cell throughout the heart such that the heart functionally behaves like a single cell or syncytium. In this way, the original neurogenic versus myogenic debate was settled in the 19th century. Many more scientists were involved in the debate than the ones mentioned here, and an excellent account of the debate and its eventual resolution has been written by Wallace Bruce Fye (1987) and it is from this account that most of the details given here have been taken.
The Professor of Genetics at Harvard Medical School, David Reich, recently wrote, “An important strand in continental European philosophy beginning in the eighteenth century was that the march of ideas proceeds in a “dialectic”: a clash of opposed perspectives that leads to a synthesis. The dialectic begins with a “thesis”, followed by an “antithesis”. Progress is achieved through a resolution, or “synthesis,” which transcends the two-sided debate that engendered it” (Reich, 2018). The original neurogenic versus myogenic debate fits this pattern. What about the “synthesis” following the debate? Of course, we now know that the heartbeat is initiated by pacemaker activity in the heart itself and the sinus node is the heart’s natural pacemaker in keeping with the myogenic theory. However, we now also know that
the heartbeat of some lower invertebrates such as decapod crustaceans and hirudinid leeches is neurogenic (Calabrese et al., 2016) and of course we know that in myogenic hearts the rate of the heartbeat initiated in the sinus node is regulated by the autonomic nervous system.
The 21st century debate
There is now a new dialectic, a new neurogenic versus myogenic debate, which has parallels with the 19th century debate. Of course, we all know that the heart rate is controlled by the autonomic nervous system – if we begin to run, the heart rate immediately increases and if we immerse our face in cold water the heart rate immediately decreases (the “diving reflex”). However, the heart rate varies chronically in other circumstances and this is the focus of the debate.
It is well known that athletes have a low resting heart rate – elite cyclists have been documented to have a resting heart rate of around 30 beats/min (D’Souza et al., 2015). For many decades in the 20th and 21st centuries, this has been commonly attributed to the autonomic nervous system and, in particular, to high vagal tone and this view is still widespread. An advocate of this neurogenic theory was the eminent British physiologist John H Coote (Al-Ani et al., 1996). It is also well known that the resting heart rate varies in a circadian manner from day to night and the resting heart rate is low at night. This is especially true in athletes whose heart rate is already low, and athletes have “nocturnal pauses” at night – the longest nocturnal pause documented in the literature is 15 seconds in a veteran athlete (Northcote et al., 1989). 15 seconds is a long time to wait for your next heartbeat! The circadian rhythm in the resting heart rate is again attributed to the autonomic nervous system and, in particular, to high vagal tone at night. As an example, a recent advocate of the neurogenic theory as applied to the circadian rhythm in the resting heart rate is the chronobiologist David Bechtold (West et al., 2017).
The neurogenic theory in the 21st century debate can therefore be considered the “thesis” in the current dialectic. Over about a decade, we have developed the “antithesis”, a voyage we have found fraught with controversy. Our involvement began with an investigation of heart rate variability, which is widely used as a surrogate measure of autonomic nerve activity (sympathetic and parasympathetic) to the heart – on the day of writing this article, a PubMed search using the term ‘heart rate variability’ returned 26,075 papers demonstrating the popularity of the technique. We showed that heart rate variability is primarily affected by the heart rate and to conclude anything about a factor independent of heart rate is difficult or impossible (Monfredi et al., 2014).
We debated the use of heart rate variability as a measure of autonomic tone in the pages of The Journal of Physiology (Boyett et al., 2019; Malik et al., 2019). Heart rate variability measurement has been the principal technique used to support the neurogenic theory of both the low resting heart rate in athletes and the circadian rhythm in the resting heart rate (Aubert et al., 2003; Sammito et al., 2016). If heart rate variability cannot be used as a measure of autonomic tone to the heart, we have to take a fresh look at the mechanisms responsible for the heart rate in athletes and the circadian rhythm in the resting heart rate. However, a second line of evidence for the neurogenic theory comes from the effects of pharmacological autonomic blockade, but this too is unconvincing because whilst some authors report that the change in heart rate is abolished (consistent with the neurogenic theory), others report it is not (Boyett et al., 2013; Black et al., 2019)!
Therefore, it is still necessary to take a fresh look at athletes and the circadian rhythm. We first investigated the resting bradycardia (decrease in resting heart rate) in exercise-trained rodents (D’Souza et al., 2014; D’Souza et al., 2017). We showed that there is a remodelling of various ion channels in the sinus node following exercise training. In particular, there is a downregulation of the important pacemaker HCN (hyperpolarisation-activated cyclic nucleotide-gated) channels, or humorously named “funny” channels, as well as the corresponding ionic (“funny”) current. By blocking the HCN channels using ivabradine we were able to abolish the difference in resting heart rate between exercise-trained and sedentary mice and we obtained data consistent with this from human athletes. The neurogenic theory of the resting bradycardia in athletes as put forward by John Coote and the myogenic theory as put forward by us was again debated in the pages of The Journal of Physiology (Coote & White, 2015; D’Souza et al., 2015). Next, we have taken a fresh look at the circadian rhythm in heart rate (unpublished data). In the mouse, we have shown that there is an intrinsic circadian rhythm in sinus node pacemaking, for example as observed in the isolated sinus node. Using RNA-seq, we have investigated the transcriptome of the sinus node over 24 hours and of 16,387 transcripts we observed a significant circadian rhythm in 44% of them (7,134 transcripts). The circadian rhythm is all pervasive and was observed in all cellular systems we looked at. For example, there is a functioning circadian clock as well as a prominent circadian rhythm in the pacemaker HCN channels, and once again by blocking the HCN channels using ivabradine we were able to abolish the difference in resting heart rate between day and night (D’Souza et al. 2020).
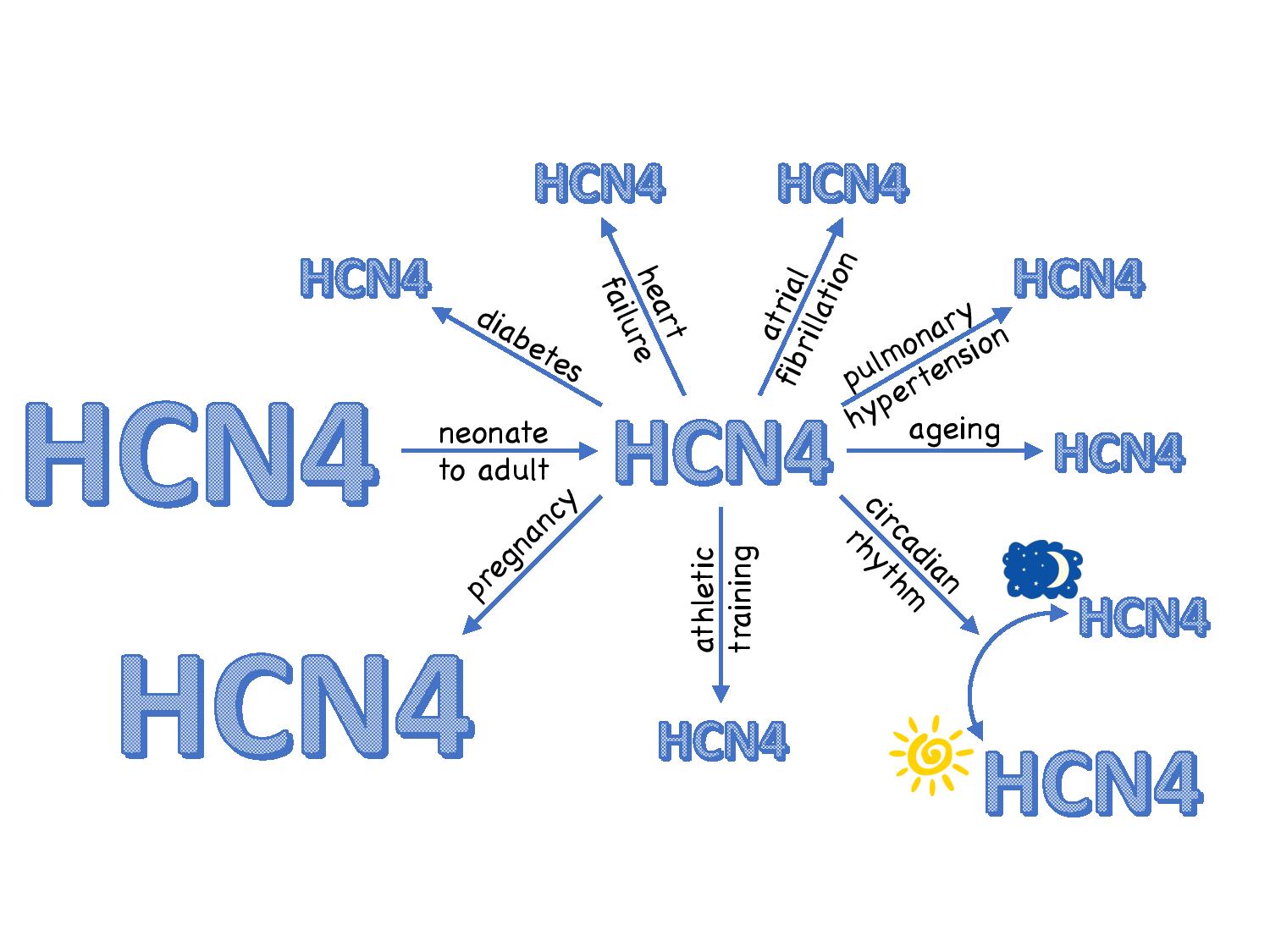
The neurogenic theory only addresses the resting bradycardia in athletes and the circadian rhythm in the resting heart rate and it does not address chronic changes in heart rate in other conditions. Such changes are widespread: the heart rate of the newborn is high and it drops as we approach adulthood. As we age, there is a decrease in the intrinsic heart rate set by the sinus node (but not the normal heart rate set by the interaction of the sinus node and autonomic nervous system). There is a 25% increase in the heart rate in the pregnant female. There is a decrease in the heart rate (or intrinsic heart rate at least) in various disease states: heart failure, pulmonary hypertension, diabetes and atrial fibrillation. In all of these cases, there are appropriate changes in the expression of HCN channels (and/or the density of the funny current) that can explain the change in heart rate (Fig. 2). Therefore, the changes in funny current and/or HCN channels in athletes and during the circadian rhythm are not unique.
It is too early for there to be a resolution of the 21st century neurogenic versus myogenic debate concerning the heartbeat. Of course, it is also too early for there to be a “synthesis”. We privately discuss whether the circadian rhythm in the resting heart rate involves the autonomic nervous system as well as intrinsic mechanisms in the sinus node. However, the role of the autonomic nervous system may be different from the traditional one and the autonomic nervous system may be controlling gene transcription – there is early evidence for this line of reasoning (Tong et al., 2013). Although our questioning of the long-standing view of the role of the autonomic nervous system in the resting bradycardia of athletes and the circadian rhythm in the resting heart rate has proved to be very unpopular (as we know from vehement opposition during peer review!), we realise “the march of ideas proceeds in a ‘dialectic’” and are therefore encouraged in persisting in our efforts!
References
Al-Ani M et al. (1996). Changes in R-R variability before and after endurance training measured by power spectral analysis and by the effect of isometric muscle contraction. European Journal of Applied Physiology and Occupational Physiology 74, 397-403. https://doi. org/10.1007/BF02337719
Aubert AE et al. (2003). Heart rate variability in athletes. Sports Medicine 33, 889-919. https://doi. org/10.2165/00007256-200333120-00003
Black N et al. (2019). Circadian rhythm of cardiac electrophysiology, arrhythmogenesis, and the underlying mechanisms. Heart Rhythm 16, 298-307. https://doi.org/10.1016/j.hrthm.2018.08.026
Boyett MR et al. (2019). CrossTalk opposing view: Heart rate variability as a measure of cardiac autonomic responsiveness is fundamentally flawed. The Journal of Physiology 597, 2599-2601. https://doi. org/10.1113/JP277501
Boyett MR et al. (2013). Viewpoint: Is the resting bradycardia in athletes the result of remodelling of the sinoatrial node rather than high vagal tone? Journal of Applied Physiology 114, 1351-1355. https://doi. org/10.1152/japplphysiol.01126.2012
Calabrese RL et al. (2016). The neural control of heartbeat in invertebrates. Current Opinion in Neurobiology 41, 68-77. https://doi.org/10.1016/j. conb.2016.08.004
Coote JH & White MJ (2015). CrossTalk proposal: bradycardia in the trained athlete is attributable to high vagal tone. The Journal of Physiology 593, 1745- 1747. https://doi.org/10.1113/jphysiol.2014.284364
D’Souza A et al. (2014). Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nature Communications 5, 3775. https://doi.org/10.1038/ncomms4775
D’Souza A et al. (2017). Targeting miR-423-5p reverses exercise training-induced HCN4 channel remodeling and sinus bradycardia. Circulation Research 121, 1058-1068. https://doi.org/10.1161/ CIRCRESAHA.117.311607
D’Souza A et al. (2015). CrossTalk opposing view: Bradycardia in the trained athlete is attributable to a downregulation of a pacemaker channel in the sinus node. The Journal of Physiology 593, 1749-1751. https://doi.org/10.1113/jphysiol.2014.284356
D’Souza A et al. (2020). A circadian clock in the sinus node mediates day-night rhythms in Hcn4 and heart rate. Heart Rhythm (in press).
Fye WB (1987). The origin of the heartbeat: A tale of frogs, jellyfish, and turtles. Circulation 76, 493-500. https://doi.org/10.1161/01.CIR.76.3.493
Gaskell WH (1880). On the tonicity of the heart and blood vessels. The Journal of Physiology 3, 48-92 16. https://doi.org/10.1113/jphysiol.1880.sp000083
Gaskell WH (1882). Preliminary observations on the innervation of the heart of the tortoise. The Journal of Physiology 3, 369-379. https://doi.org/10.1113/ jphysiol.1882.sp000110
Gaskell WH (1883). On the innervation of the heart, with especial reference to the heart of the tortoise. The Journal of Physiology 4, 43-230.14. https://doi. org/10.1113/jphysiol.1883.sp000121
Legallois CSJJ (1812). Expériences sur le principe de la vie, notamment sur celui des mouvemens du coeur, et sur le siège de ce principe; suivies du rapport fait à la première classe d l’Institut sur celles relatives aux mouvemens du coeur. D’Hautel, libraire, Paris.
Malik M et al. (2019). CrossTalk proposal: Heart rate variability is a valid measure of cardiac autonomic responsiveness. The Journal of Physiology 597, 2595- 2598. https://doi.org/10.1113/JP277500
Monfredi O et al. (2014). Biophysical characterisation of the under-appreciated and important relationship between heart rate variability and heart rate. Hypertension 64, 1334-1343.
Northcote RJ et al. (1989). Electrocardiographic findings in male veteran endurance athletes. British Heart Journal 61, 155-160. https://doi.org/10.1161/ HYPERTENSIONAHA.114.03782
Reich D (2018). Who We Are and How We Got Here: Ancient DNA and the New Science of the Human Past. Pantheon Books.
Sammito S et al. (2016). The circadian rhythm of heart rate variability. Biological Rhythm Research 47, 717-730. https://doi.org/10.3109/07420528.201 2.674592
Tong M et al. (2013). Circadian expressions of cardiac ion channel genes in mouse might be associated with the central clock in the SCN but not the peripheral clock in the heart. Biological Rhythm Research 44, 519-530. https://doi.org/10.1080/09291016.2012.704801
West AC et al. (2017). Misalignment with the external light environment drives metabolic and cardiac dysfunction. Nature Communications 8, 417. https:// doi.org/10.1038/s41467-017-00462-2