Physiology News Magazine
ATP synthesis during ischaemic muscle contractions
Features
ATP synthesis during ischaemic muscle contractions
Features
Ian R Lanza & Jane A Kent-Braun
Muscle Physiology Laboratory, Department of Kinesiology, University of Massachusetts, USA
https://doi.org/10.36866/pn.69.30
The integrity and function of skeletal muscle cells depend upon an adequate supply of adenosine triphosphate (ATP). In contracting skeletal muscle, ATP is consumed at three major sites:
- Na+-K+ ATPase, which restores the electrochemical gradient across the muscle membrane following depolarization;
- actin-myosin ATPase, which consumes ATP during crossbridge cycling in the process of force generation; and
- sarcoplasmic reticulum (SR) ATPase, which is responsible for calcium resequestration into the SR during muscle relaxation.
Skeletal muscle has a limited storage capacity for ATP, which is somewhat surprising given how crucial ATP is to cellular function. For example, during intense muscle contractions, where the consumption of ATP increases ~100-fold compared to rest, the ATP reserve would be exhausted in a matter of seconds. Remarkably, intracellular ATP concentration remains stable even after several minutes of maximumintensity muscle contractions. This phenomenon can be explained by the action of three major pathways that provide ATP at a rate that satisfies the energetic demands of muscle across a range of conditions (Fig. 1).

The ability to generate ATP oxidatively becomes compromised under conditions of limited oxygen delivery or decreased functionality of mitochondria. Clinically relevant examples of these conditions include peripheral vascular disease and mitochondrial myopathy, both of which are characterized by impaired oxidative ATP synthesis. Much research has been dedicated to understanding how ATP synthesis is affected by limitations in oxygen supply and delivery to working skeletal muscle. Studies of healthy human skeletal muscle under hypoxic or ischaemic conditions have provided some insight into the energetic response to conditions in which oxidative phosphorylation is impaired.
One could anticipate that if oxidative phosphorylation is suppressed, then intracellular ATP stores might be depleted during contractions if the demand for ATP exceeds the supply from non-oxidative pathways. Some research supports this notion by demonstrating depletion of intracellular ATP stores during ischaemic muscle contractions compared to contractions with intact blood flow. However, work from our laboratory (Lanza et al. 2006) and others (Hogan et al. 1996) has shown that cytosolic [ATP] remains unchanged during free-flow and ischaemic contractions, suggesting that skeletal muscle has a strategy for balancing ATP supply and demand even when the mitochondria are rendered incapable of generating ATP.
Does skeletal muscle compensate by increasing ATP production through other synthesis pathways, for example anaerobic glycolysis? This response is biochemically intuitive, since several substrates involved in the reduction of pyruvate to lactate (e.g. pyruvate, NADH) will accumulate in the muscle if mitochondrial flux is inhibited. Although the reduction of pyruvate to lactate is not itself an ATPgenerating step in anaerobic glycolysis, increased lactate production may indirectly help drive upstream reactions by maintaining a high cytosolic NAD+/ NADH ratio. Phosphorous magnetic resonance spectroscopy (31P-MRS) is an ideal tool for addressing questions related to muscle ATP production in vivo, as it is a non-invasive technique that allows quantification of ATP synthesis with a temporal resolution unmatched by more traditional techniques, such as muscle biopsy analyses (Kemp et al. 1994). This approach has been used to examine ATP synthesis in maximallycontracting skeletal muscle under free-flow conditions and with blood flow occluded (Conley et al. 1998; Lanza et al. 2006). In both of these studies, glycolytic flux did not increase during ischaemia, dispelling the notion, at least during brief maximal contractions with acute ischaemia, that glycolytic ATP synthesis compensates for acute limitations in mitochondrial ATP flux. Alternatively, the balance between ATP supply and demand may be met by decreasing demand for ATP rather than with a compensatory increase in ATP supply through an alternative pathway (Lanza et al. 2006). At least two mechanisms by which ATP demand may decrease during contractions in oxygen-limited conditions have been proposed (Hogan et al. 1996; Lanza et al. 2006).
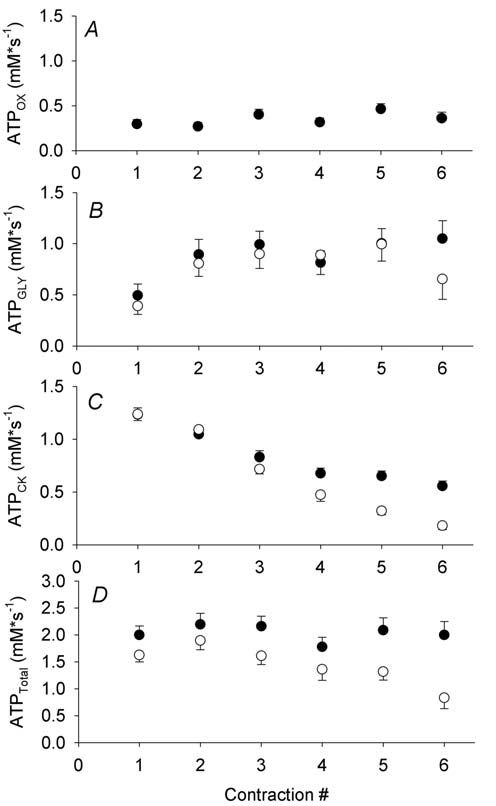
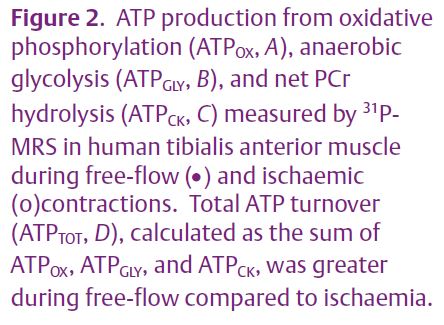
First, the demand for ATP may be tempered by increased contractile economy (force produced per unit of ATP consumed) when oxygen levels are not adequate to sustain oxidative metabolism. Using 31P-MRS measures of ATP synthesis rates (Figs. 2A, B, C), along with simultaneous measures of muscle force production, we found that contractile economy was higher in vivo during ischaemic compared with free-flow contractions in human skeletal muscle (Fig. 2D; Lanza et al. 2006). Using more invasive measures of blood flow, peripheral oxygen uptake, and ATP production, Krustrup et al. (2003) found higher mechanical efficiency and lower heat production during ischaemia than when blood flow was intact. Thus, there exists in vivo and in situ evidence to support the greater economy of ischaemic compared to oxygenated contractions. Numerous factors could contribute to increased contractile economy in the absence of adequate oxygenation; for example, changes in intracellular pH, calcium, temperature, and free phosphate have all been suggested to affect contractile economy, possibly through direct effects on the rate of cross-bridge cycling.
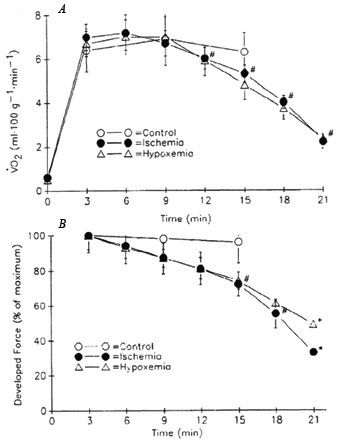
In addition to the metabolic savings conferred by increased contractile economy during ischaemia, the demand for ATP may also decrease consequent on the greater muscle fatigue during ischaemic compared to free-flow contractions. As contractility declines during fatigue, so too does the amount of ATP required by the ATPases, particularly myosin ATPase. It has been suggested that this may serve as a protective mechanism designed to prevent declines in cellular (ATP) and disruption of cellular homeostasis. Under ischaemic conditions, greater fatigue may develop due to the accumulation of intracellular metabolites (H+, Pi, H2PO4-) that are known to interfere with sarcolemmal excitability, calcium handling by the SR, and myofibrillar force generation. However, there are some intriguing data to suggest that, under certain conditions, force production is tightly coupled to oxidative ATP supply. Hogan and colleagues demonstrated this concept nicely in a perfused dog muscle preparation by imposing gradual, stepwise reductions in oxygen delivery and observing that force production decreased in parallel with declines in muscle VO2 (Hogan et al. 1996; see Fig. 3A, B). Furthermore, this response occurred in the absence of any differences in muscle lactate production, ATP depletion, or accumulation of inhibitory metabolites. This behaviour in skeletal muscle is similar to that observed repeatedly in cardiac tissue. Preconditioning heart muscle with brief bouts of ischaemia leads to decreased ATP demand in concert with reduced ATP production during subsequent bouts of prolonged ischaemia. This phenomenon, known as ‘myocardial hibernation’, can reduce subsequent infarct size by preventing severe disruptions in cellular homeostasis. The work of Hogan and others suggests that skeletal muscle is also capable of this type of behaviour.
In summary, it appears that skeletal muscle is adept at ensuring a balance between energy supply and demand under a variety of conditions, including situations where limited oxygen delivery precludes oxidative synthesis of ATP. Although compensatory increases in nonoxidative ATP production seem logical, the results from several studies suggest that this does not occur in vivo. Instead, an energetic balance appears to be accomplished by decreasing ATP demand during contractions, potentially by increasing contractile economy or decreasing force production.
References
Conley KE, Kushmerick MJ & Jubrias, SA (1998). Glycolysis is independent of oxygenation state in stimulated human skeletal muscle in vivo. J Physiol 511, 935–945.
Hogan MC, Kurdak SS & Arthur, PG (1996). Effect of gradual reduction in O2 delivery on intracellular homeostasis in contracting skeletal muscle. J Appl Physiol 80, 1313–1321.
Kemp GJ, Thompson CH, Barnes PR, & Radda GK (1994). Comparisons of ATP turnover in human muscle during ischemic and aerobic exercise using 31P magnetic resonance spectroscopy. Magn Reson Med 31, 248–258.
Krustrup P, Ferguson RA, Kjaer M, & Bangsbo J (2003). ATP and heat production in human skeletal muscle during dynamic exercise: higher efficiency of anaerobic than aerobic ATP resynthesis. J Physiol 549, 255–269.
Lanza IR, Wigmore DM, Befroy DE, & Kent-Braun JA (2006). In vivo ATP production during free-flow and ischaemic muscle contractions in humans. J Physiol 577, 353–367.