
Physiology News Magazine
Axonal filtering as a mechanism of deep brain stimulation in Parkinson’s disease
Deep brain stimulation (DBS) in Parkinson’s disease is a telling example of how the understanding of the basic principles governing basal ganglia function in the normal and diseased brain provided the rationale for a precisely targeted electro-invasive therapy. Nevertheless, the exact beneficial mechanisms of DBS at the cellular, synaptic and systemic level remain elusive. Our research emphasizes attenuation of axonal excitability as an important mechanism of DBS to abrogate pathological firing patterns.
Features
Axonal filtering as a mechanism of deep brain stimulation in Parkinson’s disease
Deep brain stimulation (DBS) in Parkinson’s disease is a telling example of how the understanding of the basic principles governing basal ganglia function in the normal and diseased brain provided the rationale for a precisely targeted electro-invasive therapy. Nevertheless, the exact beneficial mechanisms of DBS at the cellular, synaptic and systemic level remain elusive. Our research emphasizes attenuation of axonal excitability as an important mechanism of DBS to abrogate pathological firing patterns.
Features
Fang Zheng(1), Jens Volkmann(2), & Christian Alzheimer(1)
1: Institute of Physiology and Pathophysiology, University of Erlangen-Nürnberg, Erlangen, Germany
2: Department of Neurology, University of Würzburg, Würzburg, Germany
https://doi.org/10.36866/pn.85.18



Neurophysiological recordings from basal ganglia in Parkinson’s disease (PD) and in animal models thereof showed that the progressive loss of dopaminergic neurons in the substantia nigra pars compacta, which represents the neuropathological hallmark of PD, leads to abnormally synchronized burst firing and oscillations (Rivlin-Etzion et al. 2006). Most importantly, neurons of the subthalamic nucleus (STN), which are the only glutamatergic neurons of the basal ganglia, appeared to be located in a prime position to drive and maintain these pathological firing patterns. Consequently, surgical ablation of STN produced strong relief from the cardinal motor symptoms of PD, comprising akinesia, rigidity and tremor. Lending strong support to the notion that inactivation of STN neurons is essential to improve motor deficits in PD, stereotactic microinjection of the local anaesthetic lidocaine into the STN reversed the motor symptoms of PD patients (Levy et al. 2001).
In contrast to the irreversible tissue damage of surgical lesion and the transient effect of drug microinjection, electrical stimulation of STN neurons by implanted microelectrodes affords a reversible, but lasting method to modify aberrant discharge activity in the basal ganglia circuitry of PD patients. Although introduced in the 1990s and now widely employed as an effective electro-invasive therapy, it is still debated how DBS achieves its therapeutic benefits (Hammond et al. 2008; Deniau et al. 2010; Krack et al. 2010). Notably, DBS is only effective if administered at high frequency (~130 Hz), and the improvement of motor deficits displays a rapid onset and decline with the make and break of STN stimulation, respectively.
Since STN-DBS strongly mimicked the therapeutic effects of either neurosurgical ablation or lidocaine injection in PD patients, it seemed at first plausible to assume that the high-frequency electrical stimulation produces an almost immediate and quickly reversible inhibition of STN neurons, most probably involving a depolarization block of intrinsic excitability (Beurrier et al. 2001). However, the original notion that DBS stymies all neuronal activity within the STN was challenged by recordings from PD patients, in whom DBS failed to silence STN neurons (Carlson et al. 2010). Further contradiction was gathered at the other end of the experimental scale, where patch-clamp recordings from rodent STN neurons showed that these cells were well capable of following high-frequency stimulation with single action potential firing. What was strongly compromised, however, was the ionic mechanism generating burst discharges. Thus, it was argued that DBS reinstates physiological firing by ‘jamming’ pathological burst discharges (Do & Bean, 2003).
Whereas these studies were devoted to the local effects of DBS within the STN, it remained to be determined to what extent the high-frequency electrical stimuli delivered to the STN are transmitted to other regions within and even outside the basal ganglia. Remember, for example, that the STN is not only part of the ‘indirect’ pathway of the basal ganglia, but also receives a ‘hyperdirect’ corticosubthalamic input that might be activated antidromically by DBS. Within the basal ganglia network, STN-DBS might activate target neurons in the substantia nigra pars compacta, thereby possibly driving dopaminergic neurons, as well as target neurons in the output structures (internal globus pallidus and substantia nigra pars reticulata), thereby modifying central motor controls. Although experimental evidence for such network effects has been advanced (Deniau et al. 2010), concerns remain as to whether axons will faithfully conduct the high frequency of DBS over time.
To explore this issue in a preparation that allowed us to directly examine axonal excitability, we used a rat brain slice preparation preserving the STN and its projections to the substantia nigra and the entopeduncular nucleus, which is the rodent equivalent of the internal globus pallidus (Zheng et al. 2011). By placing the stimulating electrode in the STN and one recording electrode also in the STN or in one of its target regions, we were able to monitor firing activity of antidromically activated STN neurons as well as axonal conduction and synaptic transmission in orthodromically activated target regions before, during and after high-frequency stimulation (Figs 1C and 2C).

When recording single unit activity from an antidromically activated STN neuron, high-frequency stimulation did not abrogate its spontaneous firing, but modified its burst firing pattern towards shorter burst durations and longer inter-burst intervals (Fig. 1A, lower trace). This observation is consistent with the above notion that DBS does not silence STN neurons, but impairs their propensity to generate bursts.
In orthodromically connected neurons of the substantia nigra pars compacta, whole-cell patch-clamp recordings demonstrated a rapid decline of postsynaptic currents during high-frequency stimulation in the STN (Fig. 1B). Did this striking run-down result from the exhaustion of synaptic mechanisms or rather from conduction failure in afferent fibres? We resolved this issue by directly recording isolated fibre volleys, which are field potentials generated by axonal action potentials (Fig. 2). As shown in Fig. 2A and B, fibre volleys arising in different projections all rapidly declined during high-frequency STN stimulation. These data identified axonal failure as an important mechanism of DBS. Axonal failure uncouples the STN from upstream and downstream regions, thereby shielding the network against aberrant firing.
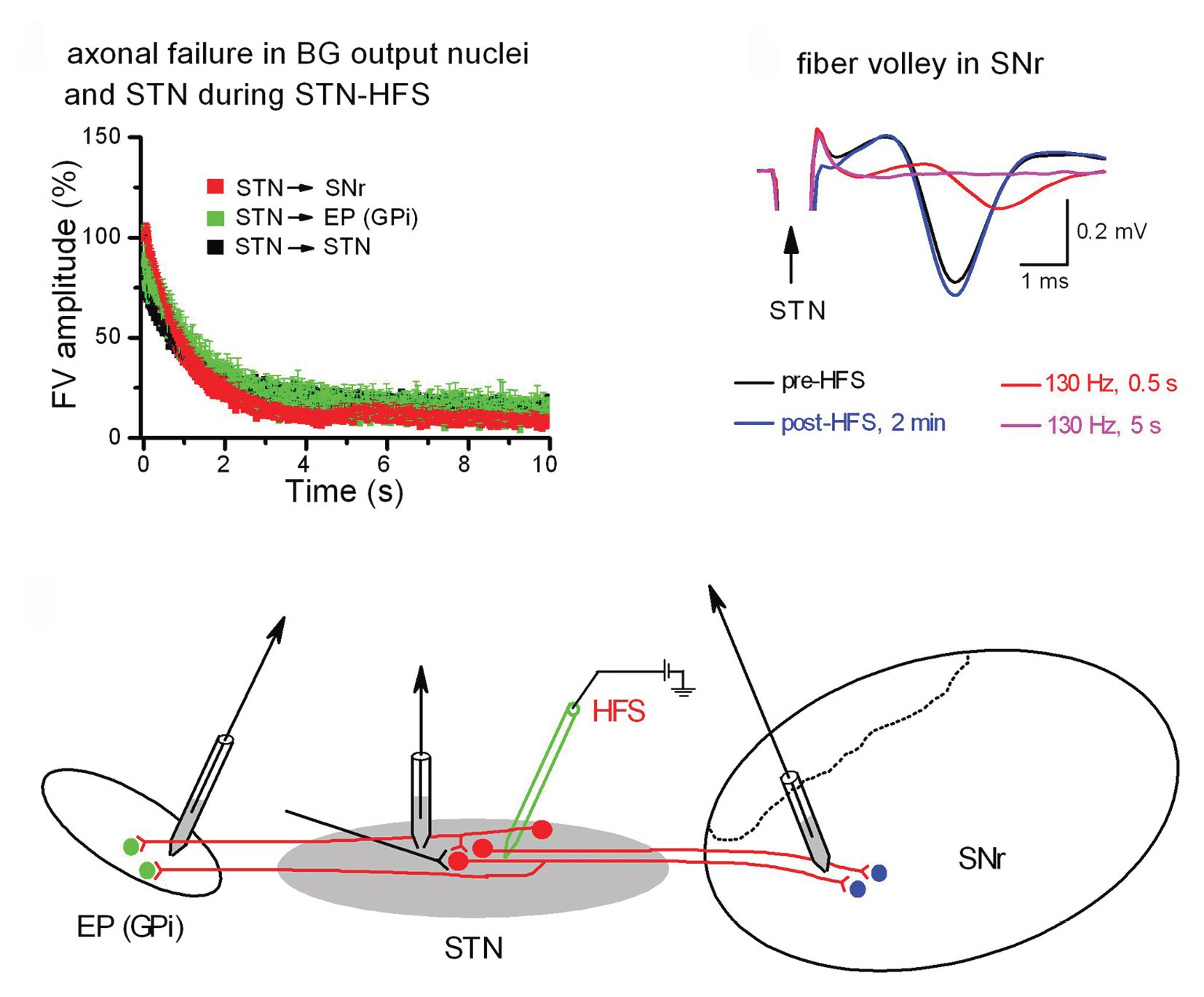
Why does DBS overburden the capacity of axons to transmit signals at high frequency? Model calculations (Bellinger et al. 2008) and our own experimental data favour a scenario in which accumulation of K+ in the submyelin space surrounding the axon and the concomitant depolarization render axonal Na+ channels inactivated. As axonal firing is progressively abating, however, K+ levels should fall again and Na+ channels should partially recover, explaining the intermittent synaptic responses that we observed during maintained high-frequency stimulation in STN target regions. Thus, DBS does not simply shut down incoming and outgoing projections to and from the STN as well as fibres of passage, but rather imposes a filter that eliminates pathological firing. By emphasizing axonal filtering as the prevailing mechanism of DBS, our research should reconcile the apparent discrepancy between the original concept that DBS locally inhibits the STN akin to surgical lesion with more recent data demonstrating remote effects in the basal ganglia network.
References
Bellinger SC, Miyazawa G & Steinmetz PN (2008). Submyelin potassium accumulation may functionally block subsets of local axons during deep brain stimulation: a modeling study. J Neural Eng 5, 263–274.
Beurrier C, Bioulac B, Audin J & Hammond C (2001). High-frequency stimulation produces a transient blockade of voltage-gated currents in subthalamic neurons. J Neurophysiol 85, 1351–1356.
Carlson JD, Cleary DR, Cetas JS, Heinricher MM & Burchiel KJ (2010). Deep brain stimulation does not silence neurons in subthalamic nucleus in Parkinson‘s patients. J Neurophysiol 103, 962–967.
Deniau JM, Degos B, Bosch C & Maurice N (2010). Deep brain stimulation mechanisms: beyond the concept of local functional inhibition. Eur J Neurosci 32, 1080–1091.
Do MT & Bean BP (2003). Subthreshold sodium currents and pacemaking of subthalamic neurons: modulation by slow inactivation. Neuron 39, 109–120.
Hammond C, Ammari R, Bioulac B & Garcia L (2008). Latest view on the mechanism of action of deep brain stimulation. Mov Disord 23, 2111–2121.
Krack P, Hariz MI, Baunez C, Guridi J & Obeso JA (2010). Deep brain stimulation: from neurology to psychiatry? Trends Neurosci 33, 474–484.
Levy R, Lang AE, Dostrovsky JO, Pahapill P, Romas J, Saint-Cyr J, Hutchison WD & Lozano AM (2001). Lidocaine and muscimol microinjections in subthalamic nucleus reverse Parkinsonian symptoms. Brain 124, 2105–2118.
Rivlin-Etzion M, Marmor O, Heimer G, Raz A, Nini A & Bergman H (2006). Basal ganglia oscillations and pathophysiology of movement disorders. Curr Opin Neurobiol 16, 629–637.
Zheng F, Lammert K, Nixdorf-Bergweiler BE, Steigerwald F, Volkmann J & Alzheimer C (2011). Axonal failure during high frequency stimulation of rat subthalamic nucleus. J Physiol 589, 2781–2793. http://jp.physoc.org/content/589/11/2781.long
