
Physiology News Magazine
Bidirectional synaptic plasticity and motor learning in the cerebellum
The learning of motor skills – fine movement control – depends upon the conversion of synaptic activity in the cerebellum into longer term changes in synaptic efficacy. Here Françis Crépel and Armelle Rancillac describe how stellate cells play a part
Features
Bidirectional synaptic plasticity and motor learning in the cerebellum
The learning of motor skills – fine movement control – depends upon the conversion of synaptic activity in the cerebellum into longer term changes in synaptic efficacy. Here Françis Crépel and Armelle Rancillac describe how stellate cells play a part
Features
Armelle Rancillac & Françis Crépel
UMR CNRS, Paris, France
https://doi.org/10.36866/pn.55.20

Synaptic plasticity and motor learning
Classically, the cerebellum is considered as being devoted to postural adjustments and motor control. In keeping with this view, the geometrical organization of the neuronal network within the cerebellar cortex (see Fig. 1) and the apparent stereotyped properties of its main cellular elements made neurobiologists believe until the 1960s that the cerebellum was a hard wired ‘neuronal machine’ exquisitely adapted to motor control (Ito, 1984). However, because motor skills can be learnt, the question arose as to whether the cerebellum is able to perform this task, despite its apparent rigid and almost crystalline structure.

As can be seen in Fig. 1, Purkinje cells (PCs) are the only output neurons of the cerebellar cortex. In the late 60s, in keeping with the then current views attributing learning and memory to enduring changes in synaptic efficacy in specific neuronal circuits, Marr and Albus proposed a theory which predicts that when parallel fibres (PFs) and climbing fibres (CFs), i.e. the two excitatory afferents to PCs, are repetitively co-activated at low rate, synapses between PFs and PCs see their efficacy durably depressed, a phenomenon called long-term depression (LTD; Fig. 1). In this system, CFs are thought to carry error signals that instruct PF synapses which were activated during incorrectly performed movements to decrease their efficacy (Ito, 1984). Following early in vivo experiments by Masao Ito and colleagues, later in vitro experiments fully confirmed this model and unravelled intracellular cascades of events leading to LTD (Daniel et al. 1998). Finally, two forms of the inverse phenomenon, i.e. long-term potentiation (LTP) of synaptic transmission between PFs and PCs, can be induced when PFs are stimulated in isolation at low rate (Fig. 2C). They differ from one another by the frequency of stimulation required for their induction, and by the underlying intracellular cascades (Lev-Ram et al. 2002).
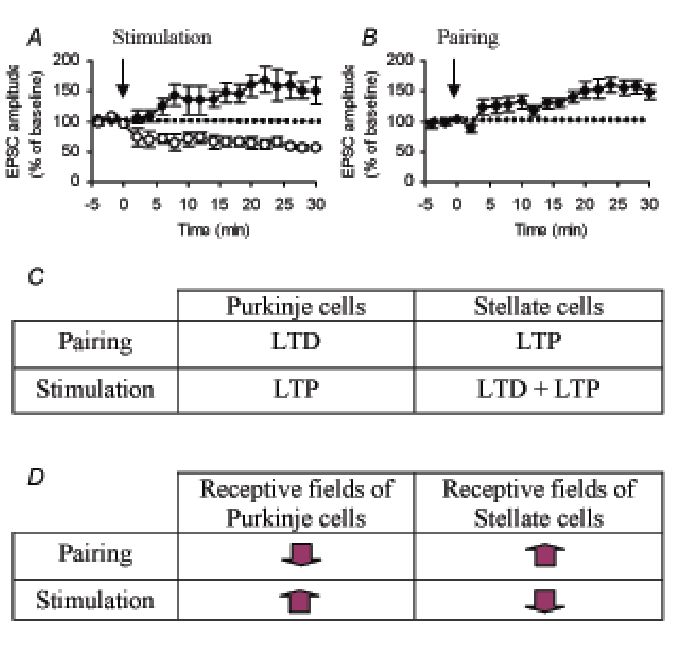
Bidirectional synaptic plasticity in cerebellar interneurones
Other putative sites of synaptic plasticity are present in the cerebellar cortex. In particular, stellate cells (SCs) are inhibitory interneurones which receive the same excitatory afferents as PCs (Fig. 1) and exert on these neurons a powerful inhibition (Ito, 1984). In principle, LTP or LTD at synapses between PFs and SCs should have a long-term counterpart on the inhibitory action exerted by these interneurones on PCs, and thus contribute to long-term changes in cerebellar output during motor learning.
In a recent series of in vitro experiments (Rancillac & Crepel, 2004), we showed that, depending on the cells, either LTP or LTD can be induced at PF-SC synapses by repetitive activation of PFs at low rate (Fig. 2A). Moreover, pairing this low frequency stimulation with post-synaptic depolarisation of SCs induced a marked shift of synaptic plasticity in favour of LTP (Fig. 2B). LTP at PF-SC synapses requires nitric oxide (NO) production as in the case of one of the two forms of LTP at PF-PC synapses (Lev-Ram et al. 2002). In contrast, LTD at PF-SC requires activation of G-protein-coupled glutamate receptors. Finally, slightly higher frequencies of PF stimulations induced a cAMP-dependent LTP at PF-SC synapses, like the second form of LTP observed at PF-PC synapses in the same conditions (Lev-Ram et al. 2002).
Thus, PF-SC synapses exhibit LTP and LTD which are very similar to those existing at PF-PC synapses with, however, a major difference. At PF-PC synapses, LTD is induced when PFs are activated at low rate in conjunction with stimulation of CFs which strongly depolarize PCs, whereas at PF-SC synapses, pairing low frequency stimulation of PFs with depolarization of SCs strongly favours LTP. Conversely, at PF-PC synapses, low frequency stimulation of PFs alone induces a NO-dependent form of LTP (Lev-Ram et al. 2002), whereas it induces LTD in half of the plastic cells at PF-SC synapses (Fig. 2C).
Relevance for motor learning
During motor learning, when co-activation of PFs and CFs leads to LTD at PF-PC synapses, the same co-activation also occurs at the level of SCs, due to collaterals of CFs impinging onto inhibitory interneurones (Fig. 1). Such co-activation is therefore likely to induce LTP at PF-SC synapses, which in turn should potentiate inhibition exerted onto PCs by SCs, and thus reinforce the decrease of responsiveness of PCs due to LTD. Such a synergy between LTP at PF-SC synapses and LTD at PF-PC synapses might therefore play an important role during motor learning. Similarly, LTD elicited at PF-SC synapses by repetitive activation of PFs alone should also work in synergy with LTP induced in PCs in the same conditions.
At a more integrated level, PCs receive information from the periphery. In particular, in the forelimb projection area of the cerebellar cortex, PCs and interneurones receive information through PFs from well delineated and rather small cutaneous areas, i.e. their so called PF cutaneous receptive fields. In an elegant series of in vivo experiments, Jörntell and Ekerot (2002) demonstrated that, in this area of the cortex, when PF are electrically stimulated alone or in conjunction with CF stimulations, unpaired PF stimulations induce long-lasting increases in the PF cutaneous receptive field sizes of PCs and induce long-lasting decreases in PF cutaneous re-ceptive field sizes of their afferent interneurones, whereas the inverse is true for paired stimulations (Fig. 2D).
This reciprocal bidirectional plasticity of PF cutaneous receptive fields of PCs and of their afferent interneurones fits very well with the bidirectional and reciprocal synaptic plasticity at PF-PC and PF-SC synapses mentioned above (Fig 2C and D). Besides their importance for understanding how information processing is managed in the cerebellum, such findings might also help to understand how the cerebellum controls motor coordination per se. Indeed, an attractive hypothesis proposes that, following signal perfor-mance errors transmitted by CFs, the corrective movement components could be initiated or driven by events that activate interneurone receptive fields and braked or terminated by events that activate PC receptive fields (Jörntell & Ekerot, 2002).
References
Jörntell H & Ekerot CF (2002). Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron 34, 797-806.
Lev-Ram V, Wong ST, Storm DR & Tsien RY (2002). A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. Proc Natl Acad Sci USA 99, 8389-8393.
Ito M (1984). The cerebellum and neural control. New-York, Raven Press, New-York.
Daniel H, Levenes C & Crepel F (1998). Cellular mechanisms of cerebellar LTD. Trends Neurosci 21, 401-407.
Rancillac A & Crepel F (2004). Synapses between parallel fibres and stellate cells express long-term changes in synaptic efficacy in rat cerebellum. J Physiol (Lond) 554, 707-720.
