
Physiology News Magazine
Bilateral interactions between the upper limbs
Richard Carson and colleagues consider the neural origins of bimanual coordination, and the implications for movement rehabilitation and therapy
Features
Bilateral interactions between the upper limbs
Richard Carson and colleagues consider the neural origins of bimanual coordination, and the implications for movement rehabilitation and therapy
Features
Richard G Carson (1), Stephan Riek (1), & Winston D Byblow (2)
1: Perception and Motor Systems Laboratory, The University of Queensland, Australia
2: Human Motor Control Laboratory, The University of Auckland, New Zealand
https://doi.org/10.36866/pn.58.37

In the course of our daily activities we routinely engage in tasks in which the two hands execute quite different actions. The apparent ease with which we unscrew the cap from a bottle, or thread a needle, belies the fact that there is a strong tendency for simultaneous movements of the upper limbs to be drawn towards one another. This predisposition is expressed clearly in a test of dexterity with which we are all familiar: rubbing your stomach and patting your head at the same time (if this seems easy at first, try swapping the role assigned to each hand). Mirror movements – involuntary muscular contractions during what are intended to be unilateral movements of the opposite limb, represent an extreme expression of this tendency. These are typically considered pathological, as they occur in association with specific disorders of the CNS. Yet they are also observed frequently in normally developing children, and bilateral motor irradiation – an increase in the excitability of the (opposite) homologous motor pathways when unilateral contractions are performed, is a robust feature of the mature motor system.
These phenomena are not simply of academic interest. The systematic nature of the interactions that occur between movements of the upper limbs has given rise to the expectation that functional improvements in the control of paretic muscles may be realised when bilateral movements are prescribed. Indeed, in both acute and chronic stroke survivors, bilateral movement training leads to improvements in unilateral actions performed subsequently by the hemiplegic arm (e.g. Whitall et al. 2000). It is clearly desirable that there are principled grounds upon which to develop programs of movement rehabilitation and therapy. Yet until recently there has been remarkably little consensus concerning the neural mechanisms that mediate bilateral interactions between the upper limbs.
Transcranial magnetic stimulation (TMS) affords a means of probing the excitability of corticospinal pathways with a degree of sensitivity beyond that which can be achieved by other non-invasive techniques. It is therefore being used with increasing frequency to examine the nature of bilateral motor irradiation. An initial finding that electromyographic (EMG) responses evoked by TMS were potentiated during forceful contractions of the opposite hand, in patients with agenesis of the corpus callosum, suggested that the interactions might occur below the level of the cortex (Meyer et al. 1995). Recent studies, however, challenge this view and reveal that in neurologically intact individuals, bilateral motor irradiation is mediated at least in part by interhemispheric interactions between cortical motor areas.
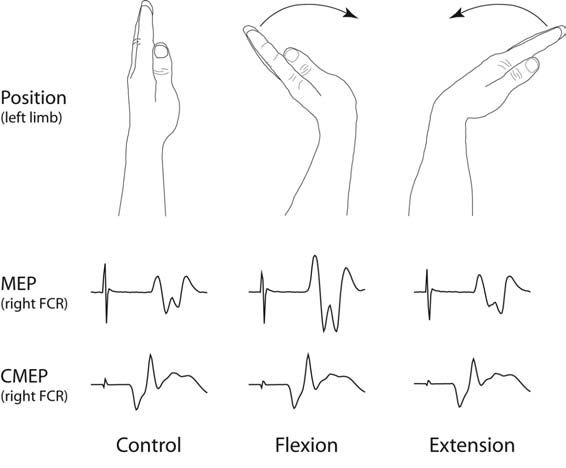
Hortobágyi et al. (2003) reported that during moderate to strong isometric contractions of the wrist flexors, potentials evoked in a homologous muscle of the opposite limb by TMS were potentiated. In contrast, those evoked by direct stimulation of the descending tracts at the level of the cervicomedullary junction (CMEPs) were unaffected by the contractions of the muscles of the opposite limb. This latter finding indicated that there was no change in the excitability of the spinal motoneurons. We have recently shown that during cyclic flexion and extension of the wrist, the largest responses to TMS are obtained during the phases of movement in which the corresponding muscle of the opposite limb is most strongly engaged (Fig. 1). Responses to cervicomedullary stimulation were unaffected by movement of the opposite limb (Carson et al. 2004). These studies indicate that the bilateral motor irradiation generated by both tonic and phasic contractions has a cortical component.
The hand and forearm representation in primary motor cortex (M1), the area most obviously implicated in movement execution, is relatively sparsely connected with its contralateral counterpart. Secondary motor areas such as the cingulate motor area (CMA), exhibit much denser connectivity via the corpus callosum. Indeed, it appears that the scope for direct inter-hemispheric interactions via callosal pathways decreases progressively along a functional gradient that culminates in those regions that have the most prominent role in generating motor output. Bilateral motor irradiation may arise first in the secondary motor areas of the hemisphere contralateral to the moving limb, spread through the callosal fibres to secondary motor areas in the opposite cortex, and subsequently to the primary motor cortex ipsilateral to the moving limb (Fig. 2).

The degree of crossed-facilitation may thus be contingent upon activity in motor centres “upstream” of M1, rather than upon the level of output from the primary motor cortex that is engaged to generate the movement. This conjecture is supported by recent studies in which it has been shown that the relationship between the motor output to the actively moving limb, and the degree of bilateral motor irradiation, is altered by the mechanical context in which the movements are performed (Carson & Riek, 2000), and when there is visual feedback of the moving limb (Carson et al. 2004).
The conclusion that bilateral motor irradiation is mediated to a significant degree by interhemispheric interactions between cortical motor areas has important implications with respect to strategies for rehabilitation. In particular, it suggests that the efficacy of bilateral training may be enhanced by the conjoint use of techniques such as sensory-induced plasticity that promote the functional remodelling of cortical motor areas. The specific brain areas that might most appropriately be the targets for these techniques, however, remain to be resolved.
Acknowledgements
This work was funded by the Australian Research Council and the National Health and Medical Research Council of Australia.
References
Carson R G & Riek S (2000). Musculo-skeletal constraints on corticospinal input to upper limb motoneurones during coordinated movements. Human Movement Science 19, 451-474.
Carson R G, Riek S, Mackey D C, Meichenbaum D P, Willms K, Forner M & Byblow W D (2004). Excitability changes in human forearm corticospinal projections and spinal reflex pathways during rhythmic voluntary movement of the opposite limb. J Physiol 560, 929-940.
Carson R G, Welsh T N & Pamblanco-Valero M-A (2004). Visual feedback alters the variations in corticospinal excitability that arise from rhythmic movements of the opposite limb. Experimental Brain Research. Published Online: 23rd October 2004. Digital Object Identifier (DOI): 10.1007/s00221-004-2076-x.
Hortobágyi T, Taylor J L, Petersen N T, Russell G & Gandevia S C.(2003). Changes in segmental and motor cortical output with contralateral muscle contractions and altered sensory input in humans. J Neurophysiol 90, 2451-2459.
Meyer B-U, Röricht S, Gräfin von Einsiedel H, Kruggel F & Weindl A (1995). Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with adnormalities of the corpus callosum. Brain 118, 429-440.
Whitall J, McCombe Waller S, Silver K H C & Macko R F (2000). Repetitive bilateral arm training with rhythmic auditory cueing improves motor function in chronic hemiparetic stroke. Stroke 31, 2390-2395.
