
Physiology News Magazine
Brain food: the cerebral metabolic response to exercise
Following intense activation of the brain its carbohydrate uptake is enhanced, suggesting to Mads Dalsgaard that central fatigue relates to depletion of cerebral stores of glycogen
Features
Brain food: the cerebral metabolic response to exercise
Following intense activation of the brain its carbohydrate uptake is enhanced, suggesting to Mads Dalsgaard that central fatigue relates to depletion of cerebral stores of glycogen
Features
Mads K. Dalsgaard
Department of Anaesthesia, Rigshospitalet Copenhagen, Denmark
https://doi.org/10.36866/pn.53.29

In order for skeletal muscles to contract a complex chain of events has to be established within the central nervous system (CNS), including generating the idea and probably subconscious planning of learned movement involving adjustment of its force and direction, before the motoneurons are addressed. Only when performing a task that has not been practiced, or when a contraction is intense or repeated to fatigue, it is conscious or directed by a ‘will’. In more complex tasks such as walking or running an even higher level of integration is required to maintain balance and to excite the cardiovascular and respiratory systems. Also, psychological factors come into play, such as verbal encouragement or critical feedback provided by the coach. Paradoxically, such external stimuli may in themselves induce fatigue as seemingly the brain has a limit to how much information it can process without compromising its ability to recruit the motor system. The influence of external stimuli on the brain’s contribution to performance was already established in 1904 as Mosso coined the phrase ‘mental fatigue’ when a colleague of his could not accomplish the usual work with a finger ergograph after giving a lecture. Similar apparent central fatigue is established when subjects, after work performed until exhaustion with the eyes closed, become able to continue the work by opening their eyes. Equally, muscle strength and endurance may be enhanced if some ‘diverting activity’, i.e. calculations or activity of a different kind, is performed at the same time.
Cerebral blood flow and metabolism in exercise
With such complex integration of motor control, it would seem that brain metabolism, and in turn its blood flow, would be enhanced with physical activity. Yet, the pioneering observation from the time of Mosso’s experiments by Atwater and Benedict which showed an increase in whole body metabolism during thinking has not been confirmed and may be ascribed to muscle tension when concentrating on a given task. With the introduction of the Kety-Schmidt technique global cerebral blood flow (gCBF) and metabolic rate of oxygen (CMRO2) were found to remain remarkably stable in all conditions. Reductions in these variables are noted during deep sleep and hypoglycaemia, while only exercise in the heat increases CMRO2. However, as demonstrated by Lassen and coworkers, such stability of gCBF and gCMRO2 during mental activity reflects marked changes in the regional values while during exercise, these increases take place in areas of the brain that are not only involved in motor control but also in integration of sensory input, and control of ventilation and cardiovascular variables. It may be that activity in one or several parts of the brain is compensated by downregulation of blood flow and metabolism in other parts of the brain. This notion would explain why it is difficult to concentrate on more than one thing at a time with respect to both intellectual performance and complex patterns of movement.
The cerebral metabolic ratio
In contrast to skeletal muscle where activity results in a disproportional increase in metabolism compared to blood flow supply (as inferred by a decrease in muscle oxygenation), CNS responds to activation with a larger increase in regional blood flow than the concomitant increase in CMRO2, as detected both by near infrared spectroscopy, positron emission tomography and functional magnetic resonance. However, activated brain tissue appears to take up glucose out of proportion to its uptake of oxygen both on a local and a global level. Mental activity and visual stimulation decrease the global cerebral metabolic ratio (gCMR; O2 uptake/glucose uptake) from the resting value of close to 6 to about 5.4 (Madsen et al. 1995).
With respect to exercise, a submaximal effort does not influence gCMR, while during maximal exercise it decreases to a low of ~ 3.7 (Dalsgaard et al. 2002) or to ~ 3 during whole body exercise, when lactate is also taken into consideration (Fig. 1). During prolonged submaximal exercise gCMR stays stable for as long as the workload seems relatively easy, but when the subject struggles to continue the work after perhaps 1–2 hours, gCMR decreases (Nybo et al. 2003). During intense exercise that requires the subject’s instant attention, or with unfamiliar exercise such as arm-cranking, gCMR decreases from the onset of exercise. Moreover, it is a consistent finding that gCMR remains low for some five minutes after exercise.
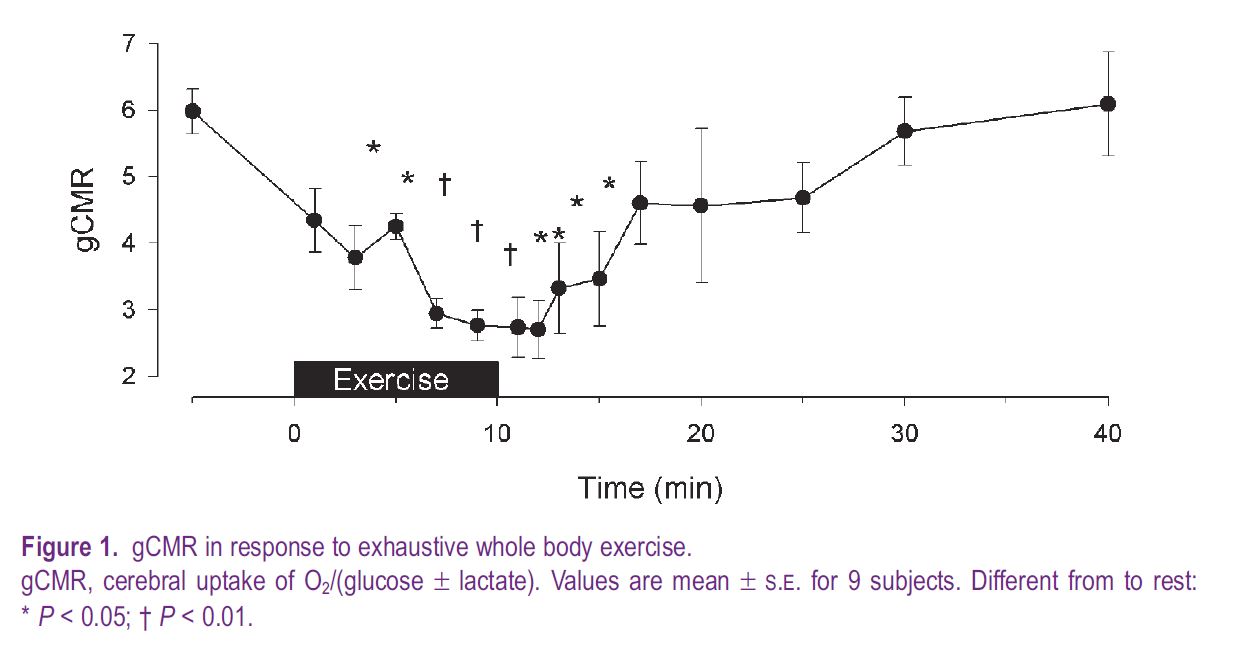
We evaluated the influence of central command on gCMR during exercise with partial neuromuscular blockade (Dalsgaard et al. 2002). During pharmacologically-induced neuromuscular blockade, exercise at a given workload requires extra effort in order to recruit more muscle and compensate for the fraction of motor units that do not contribute to the contraction. In that situation, gCMR is reduced to ~ 5, or slightly less, compared to control maximal exercise, although the effort in both conditions is maximal. The moderate reduction of gCMR during exercise with neuromuscular blockade may be explained by an influence by sensory input from the muscles. The impact of such sensory stimuli on brain metabolism is addressed when thigh cuffs impede blood flow to the legs and cause severe pain (Dalsgaard et al. 2003). During exercise with muscle ischaemia, as with postexercise muscle ischaemia, gCMR decreases to about 4.0–4.5. Thus, the very low gCMR developed during maximal exercise may reflect the combined effect of mental activity associated with central command and sensory input from the muscles on cerebral metabolism.
Energy sources for the brain
While it is evident that the gCMR decreases in response to a mental effort, including intense exercise, it is less clear why this happens. For the brain at rest oxygen and glucose are taken up in a ratio close to ~ 6, which implies that glucose undergoes complete oxidation and therefore is the primary substrate for the brain. A slightly lower gCMR of about ~ 5.7 is often noted at rest, consistent with the idea that part of the energy production results from glycolysis, which is also signified by a discrete lactate efflux from the brain. However, substrates other than glucose may serve as an energy source for the brain. Lactate becomes of value when the blood concentration increases as observed during cardiopulmonary resuscitation, insulin dependent diabetes mellitus, hypoglycaemia, and in particular when the brain is activated during exercise where lactate uptake may exceed that of glucose. Since the lactate taken up by the brain during and after exercise is not released back into the blood it is presumably metabolised. Alternatively, lactate taken up by the brain could accumulate in a distribution volume, but even after a marked cerebral uptake of lactate, it does not build up in the cerebrospinal fluid or within the brain as determined by magnetic resonance spectroscopy. Also, on a local level, cultures of brain cells, and especially neurons, metabolise lactate and it is preferred to glucose when the brain is recovering from hypoxia. In humans infusion of lactate prevents hypoglycaemic symptoms and reduces glucose metabolism.
The brain possesses the capacity to oxidise fatty acids and with their large carbon skeleton even a small cerebral uptake of fatty acids could challenge gCMR. However, with exercise the uptake of free fatty acids by the brain is negligible (Dalsgaard et al. 2002). Moreover, glycerol, glutamate, glutamine and alanine uptake from the blood is not of functional importance for whole brain metabolism during exercise. In addition, even though ketone bodies can serve as an energy source for the brain, particularly during a fast and in insulin dependent diabetes mellitus patients, this is not the case during exercise where plasma concentration remains low. Notwithstanding that the brain can utilise a variety of substrates if their availability increases, or if the supply of glucose is low, gCMR would remain stable if neuronal activity was fuelled by a balanced oxidative metabolism.
Glycogen and lactate metabolism
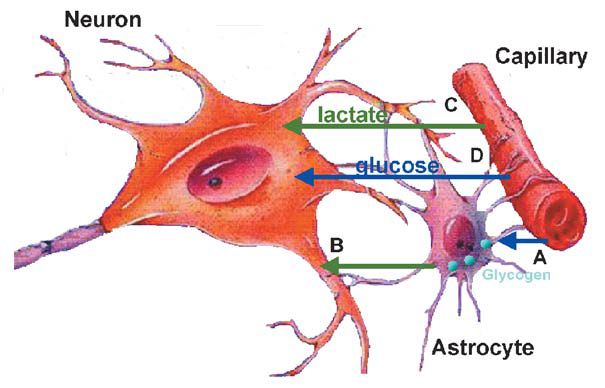
The uptake of carbohydrate in excess of oxygen, as implied by a reduced gCMR, provokes speculation as to whether the brain metabolises glycogen. Besides lactate taken up from blood, neurons may be provided with lactate released from the breakdown of glycogen in neighbouring astrocytes (Fig. 2) (Brown et al. 2003, see also page 18). With cerebral activation in the rat, the cerebral glycogen store is diminished from the resting value of ~ 6–8 µmol glucose equivalents per gram, similar to the level found during tumour surgery in humans. After such depression of brain glycogen, its replenishment may reach a higher value than at rest (‘super-compensation’) a phenomenon also known to occur in skeletal muscle during recovery from prolonged exercise and in the rat brain after hypoglycaemia. Also lack of sleep affects the cerebral glycogen level supporting the notion that glycogen metabolism and generation of lactate form an integrated part of brain metabolism.
Changes in the level of glycogen within the brain may not be the only reason why gCMR decreases in response to intense activation. Accelerated glucose uptake and metabolism are likely to depend on accumulation of intermediates. Furthermore, glucose serves as precursor for amino acids and, after activation of the brain in the rat, about half of the decrease in gCMR has been ascribed to accumulation of glutamate and other metabolites. Glutamate has been suggested to be involved in central fatigue, but that is not supported by the arterio-venous difference over the brain when gCMR is low. However, it remains a possibility that brain metabolism of amino acids does not become manifest in blood within a time frame that affects gCMR during or immediately after exercise.
gCMR and central fatigue
The implication of a reduced gCMR during neuronal activation remains unknown. Intense cerebral activity causes the energy demand to exceed the production and a reduced glycogen level in the astrocytes could influence neuronal function. Conversely, the reduced gCMR after exhaustive exercise suggests glycogen repletion. If this occurs brain glycogen deposits appear to recover within ~ 5 min, and this time frame corresponds with the observation that following exhaustive exercise athletes are willing to continue the work after only a few minutes of recovery.
References
Brown AM, Tekkok SB & Ransom BR (2003). Glycogen regulation and functional role in mouse white matter. J Physiol 549, 501-512.
Dalsgaard MK, Ide K, Cai Y, Quistorff & Secher NH (2002). The intent to exercise influences the cerebral O2/carbohydrate uptake ratio in humans. J Physiol 540, 681-689.
Dalsgaard MK, Nybo L, Cai Y & Secher NH (2003). Cerebral metabolism is influenced by muscle ischaemia during exercise in humans. Exp Physiol 88, 297-302.
Madsen PL, Hasselbalch SG, Hagemann LP, Olsen KS, Bulow J, Holm S, Wildschiodtz G, Paulson OB & Lassen NA (1995). Persistent resetting of the cerebral oxygen/glucose uptake ratio by brain activation: evidence obtained with the Kety-Schmidt technique. J Cereb Blood Flow Metab 15, 485-491.
Nybo L, Nielsen B, Blomstrand E, Moller K & Secher NH (2003). Neurohumoral responses during prolonged exercise in humans. J Appl Physiol 95, 1125-1131
