
Physiology News Magazine
Brain glycogen decrease and central fatigue during prolonged exercise
Hard physical exertion impacts not only on the body but also on the mind. The metabolic changes in neurons reveal, as in muscle, that glycogen plays a crucial role.
Features
Brain glycogen decrease and central fatigue during prolonged exercise
Hard physical exertion impacts not only on the body but also on the mind. The metabolic changes in neurons reveal, as in muscle, that glycogen plays a crucial role.
Features
Takashi Matsui & Hideaki Soya
Laboratory of Exercise Biochemistry and Neuroendocrinology, Institute of Health and Sport Sciences, University of Tsukuba, Japan
https://doi.org/10.36866/pn.87.19
Prolonged exercise can induce central fatigue, the mechanisms of which have yet to be elucidated. The definition of fatigue is a ‘failure to maintain the required or expected force’ or ‘a reduction in muscle force and/or power-generating capacity’, and should be acknowledged as a complex phenomenon influenced by both peripheral and central factors (Nybo & Secher, 2004). It is well known that depletion of skeletal muscle glycogen contributes to peripheral fatigue during exercise. The brain also has glycogen, stored in astrocytes, which is an energy source for neurons. The hypothesis that brain glycogen is used during exercise has remained untested until now.
Energy sources for the brain: astrocyte–neuron lactate shuttle
The astrocyte–neuron lactate shuttle hypothesis posits that lactate released from astrocytes into the extracellular space is metabolized by neurons (Oz et al. 2009). Until recently, only blood-borne glucose was considered an energy source for the brain. However, current studies have shown that when there is neuronal activation, lactate is released from astrocytes and the neurons consume it predominantly for energy. Furthermore, culture experiments have shown that glycogen localized in astrocytes is degraded into lactate by excitatory neurotransmitters such as noradrenaline and serotonin, and can contribute to the release of lactate from astrocytes to neurons through aerobic glycolysis (Brown, 2004).
Brain glycogen assays
Not so long ago, it was believed that the brain glycogen levels were so low that they could not play a significant role in cerebral metabolism during some physiological stimulations including exercise. Further, there are technical difficulties involved in determining the post-mortem brain glycogen levels in animal tests because brain glycogen is metabolized rapidly following death. The current optimal method is to snap-inactivate glycogen-metabolizing enzymes using high-power (10 kW) microwave irradiation (MI) (Kong et al. 2002; Matsui et al. 2011). Here, MI enabled us to inhibit glycogen metabolism after animal death by elevating brain temperature to approximately 90°C within 1 s, which allowed us to take accurate measurements of brain glycogen levels.

Physiological role of brain glycogen
Animal studies using MI have shown that astrocytic glycogen is degraded into lactate to provide fuel for neurons during hypoglycaemia, sleep deprivation and memory formation (Brown, 2004). Interestingly, neuronal activation in the cortex during hypoglycaemia was prolonged when the basal level of brain glycogen was elevated (Suh et al. 2007). Furthermore, the inhibition of hippocampal glycogen degradation in rats with 1,4-dideoxy-1,4-imino-D-arabinitol (DAB, a glycogen degradation inhibitor), interdicted long-term memory formation (Suzuki et al. 2011). Collectively, these results show that astrocytic glycogen is a critical energy source for neurons when the glucose provision from blood is insufficient and when there are sudden increases in energy demands during neuronal activation.
Brain glycogen decreases during prolonged exercise
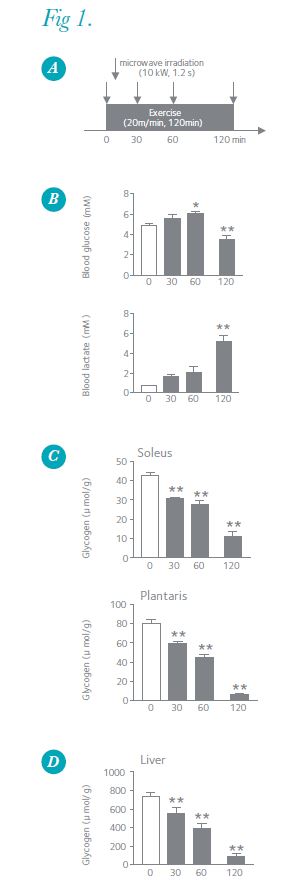
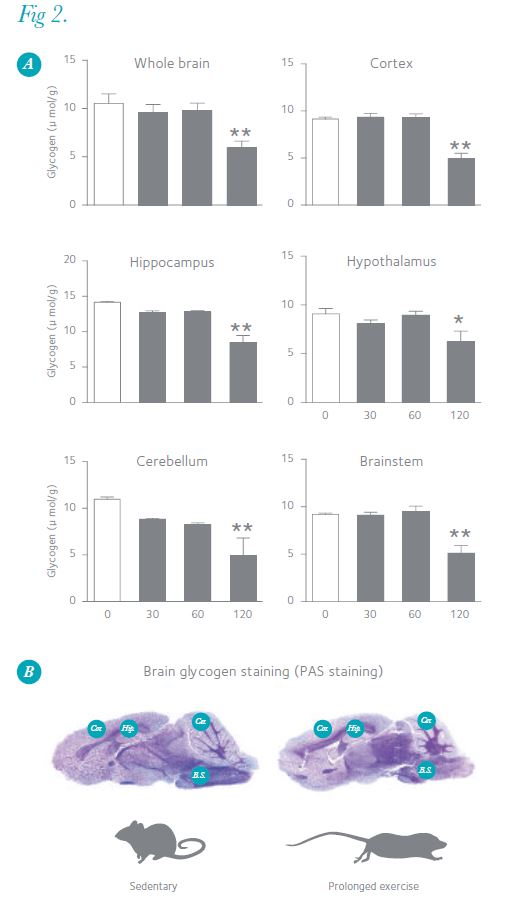
Exercise increases the brain’s energy demands through neuronal activation, and prolonged exercise induces hypoglycaemia, leading us to postulate that brain glycogen decreases during exercise. To test this hypothesis, we exercised male Wistar rats on a treadmill for different durations (30–120 min) at moderate intensity (20 m min–1) and measured their brain glycogen levels using MI (Fig. 1A). At the end of 30 and 60 min of running, blood glucose levels did not decrease compared with those of pre-exercise, but at the end of 120 min, blood glucose was 46% lower than pre-exercise levels (Fig. 1B). After 30 and 60 min, brain glycogen levels remained unchanged from resting levels, but liver and muscle glycogen decreased (Fig. 1C and D). After 120 min, brain glycogen levels decreased significantly by an average of 34–60% in five discrete brain loci (the cerebellum, 60%; cortex, 48%; hippocampus, 43%; brainstem, 37%; and hypothalamus, 34%) compared with those of pre-exercise levels (Fig. 2A). Figure 2B shows image data of brain glycogen staining, and we can see that glycogen decreases especially in the cortex, hippocampus, cerebellum and brainstem. The brain glycogen levels after running in all five regions were significantly correlated with the respective blood glucose (positive) and with brain lactate levels (negative) (data not shown). Further, in the cortex, the levels of metabolites of noradrenaline (methoxyhydroxyphenylglycol; MHPG) and serotonin (5-hydroxyindoleacetic acid; 5-HIAA), which are potentially involved in the degradation of brain glycogen, increased during prolonged exercise and negatively correlated with glycogen levels (data not shown). This supports the hypothesis that brain glycogen decreases with prolonged exhaustive exercise, and suggests that increased noradrenaline and serotonin together with hypoglycaemia are associated with glycolysis in the brain (Matsui et al. 2011).
Brain glycogen decrease and central fatigue
Brain glycogen decrease may be an integrative factor of central fatigue during prolonged exercise. Until now, hypoglycaemia with muscular and liver glycogen depletion, an increase in brain serotonin (serotonin hypothesis) and a high brain temperature (hot brain) have been recognized as factors inducing central fatigue during prolonged exercise (Newsholme et al. 1992; Nybo & Secher, 2004). These are interesting as complex phenomena under the influence of both peripheral and central factors. On the other hand, hypoglycaemia and serotonin are not only inducing factors of central fatigue but also enhancing factors of astrocytic glycogen degradation. Thus, brain glycogen decreases with prolonged exercise led us to postulate that hypoglycaemia together with brain activation with noradrenaline and/or serotonin metabolism may be involved in the development of decreased brain glycogen, and may shed light on studies examining how brain glycogen metabolism is involved in central fatigue during exercise (Fig. 3) (i.e. understanding how enhancing the effects of brain glycogen storage and availability may delay exercise-induced exhaustion).
In this article, we introduce animal experiment data regarding brain glycogen metabolism and central fatigue during prolonged exercise. A human study using nuclear magnetic resonance (NMR) also showed that brain glycogen only decreases during hypoglycaemia (Oz et al. 2009). Thus, it is possible that exercise-induced brain glycogen decrease also occurs in humans. We have also observed that brain glycogen recovers to above pre-exercise levels (‘supercompensation’) following prolonged exhaustive exercise, as does skeletal muscle glycogen (Matsui et al. 2012). It may soon be possible to propose new concepts such as ‘brain glycogen loading’ to mitigate central fatigue during exercise.

Acknowledgements
This work was supported in part by Grant-in-Aid for Challenging Exploratory Research of the Japan Society for the Promotion of Science (No. 21650166), by Grant-in-Aid for JSPS Fellows (No. 10J00513), by a grant from the Kozuki Foundation for Sports and Education, and by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) for the Body and Mind Integrated Sports Sciences (BAMIS) Project (2010). Takashi Matsui is a Research Fellow of JSPS.
References
Brown AM (2004). Brain glycogen re-awakened. J Neurochem 89, 537–552.
Kong J, Shepel PN, Holden CP, Mackiewicz M, Pack AI & Geiger JD (2002). Brain glycogen decreases with increased periods of wakefulness: implications for homeostatic drive to sleep. J Neurosci 22, 5581–5587.
Matsui T, Ishikawa T, Ito H, Okamoto M, Inoue K, Lee MC, Fujikawa T, Ichitani Y, Kawanaka K & Soya H (2012). Brain glycogen supercompensation following exhaustive exercise. J Physiol 590, 607–616.http://jp.physoc.org/content/590/3/607.long
Matsui T, Soya S, Okamoto M, Ichitani Y, Kawanaka K & Soya H (2011).Brain glycogen decreases during prolonged exercise. J Physiol 589, 3383–3393.http://jp.physoc.org/content/589/13/3383.long
Newsholme EA, Blomstrand E & Ekblom B (1992). Physical and mental fatigue: metabolic mechanisms and importance of plasma amino acids. Br Med Bull 48, 477–495.
Nybo L & Secher NH (2004). Cerebral perturbations provoked by prolonged exercise. Prog Neurobiol 72, 223–261.
Oz G, Kumar A, Rao JP, Kodl CT, Chow L, Eberly LE & Seaquist ER (2009). Human brain glycogen metabolism during and after hypoglycemia. Diabetes 58, 1978–1985.
Suh SW, Bergher JP, Anderson CM, Treadway JL, Fosgerau K & Swanson RA (2007). Astrocyte glycogen sustains neuronal activity during hypoglycemia: studies with the glycogen phosphorylase inhibitor CP-316,819 ([R-R*,S*]-5-chloro-N-[2-hydroxy-3-(methoxymethylamino)-3-oxo-1-(phenylmet hyl)propyl]-1H-indole-2-carboxamide). J Pharmacol Exp Ther 321, 45–50.
Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ & Alberini CM (2011). Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823.
