
Physiology News Magazine
Brain glycogen re-awakened
Why concern ourselves with an apparently inconsequential compound, asks Angus Brown
Features
Brain glycogen re-awakened
Why concern ourselves with an apparently inconsequential compound, asks Angus Brown
Features
Angus Brown
MRC Applied Neuroscience Group, University of Nottingham
https://doi.org/10.36866/pn.53.18

Glycogen is the main carbohydrate storage depot in the body. It is a highly branched polysaccharide of Dglucose into which excess glucose is stored, and from which glucose (or equivalent) is rapidly liberated upon demand. Glycogen is an advantageous form in which to store excess intracellular glucose, as it reduces osmotic forces on the cell, requires no ATP for initiation of metabolism, is rapidly metabolised and, unlike fatty acids, can yield ATP under anaerobic conditions. The main stores of glycogen are in the liver and skeletal muscle, but its function is dependent upon location. Liver glycogen is released as glucose into the systemic circulation in response to falling blood glucose levels, thus maintaining normoglycaemia and benefiting the entire organism. Muscle glycogen, however, is used as a local energy source solely by skeletal muscle cells during muscle contraction.
Glycogen is also present in the brain, where it is located predominately in astrocytes, but its function is unknown. It is widely accepted that brain glycogen can only support function for a few minutes in the absence of glucose, thus a role as a viable energy reserve has been universally dismissed. This assumption appears valid when one considers the amount of glycogen contained in each tissue. Resting skeletal muscle contains about 400g of glycogen (1–2% of tissue weight), well fed liver contains about 100g of glycogen (6–8% of tissue weight), but current estimates of brain glycogen are a meagre 0.5–1.5g (0.1% of tissue weight). Why concern ourselves with an apparently inconsequential compound? As ever the answer lies in the fine detail. It is the central dogma of brain energy metabolism that the brain does not contain any significant energy reserves, and is thus entirely dependent on the systemic circulation to deliver a constant, uninterrupted supply of glucose in excess of demand. Glucose is not the only bloodborne metabolite, but the limiting permeability of the blood brain barrier excludes potential metabolites from the brain. However, a variety of in vitro brain preparations, in which bloodborne delivery of metabolites is circumvented, can survive on nonglucose metabolites. These include, but are not limited to, the sugars mannose and fructose, the monocarboxylates lactate and pyruvate, and the ketone bodies β-hydroxybutyrate and acetoacetate. Glucose-derived metabolites generated within the brain parenchyma are therefore potential substrates for oxidation. The question of non-glucose metabolites serving as nutrients for neurones is highly controversial (Chih et al. 2001; Vannucci & Simpson, 2001). The diplomatic view is that it is inconceivable/highly likely* that the brain utilises glucose-derived metabolites, thus a prominent role for glycogen in supporting brain function is inconceivable/a realistic possibility* (* delete accordingly).
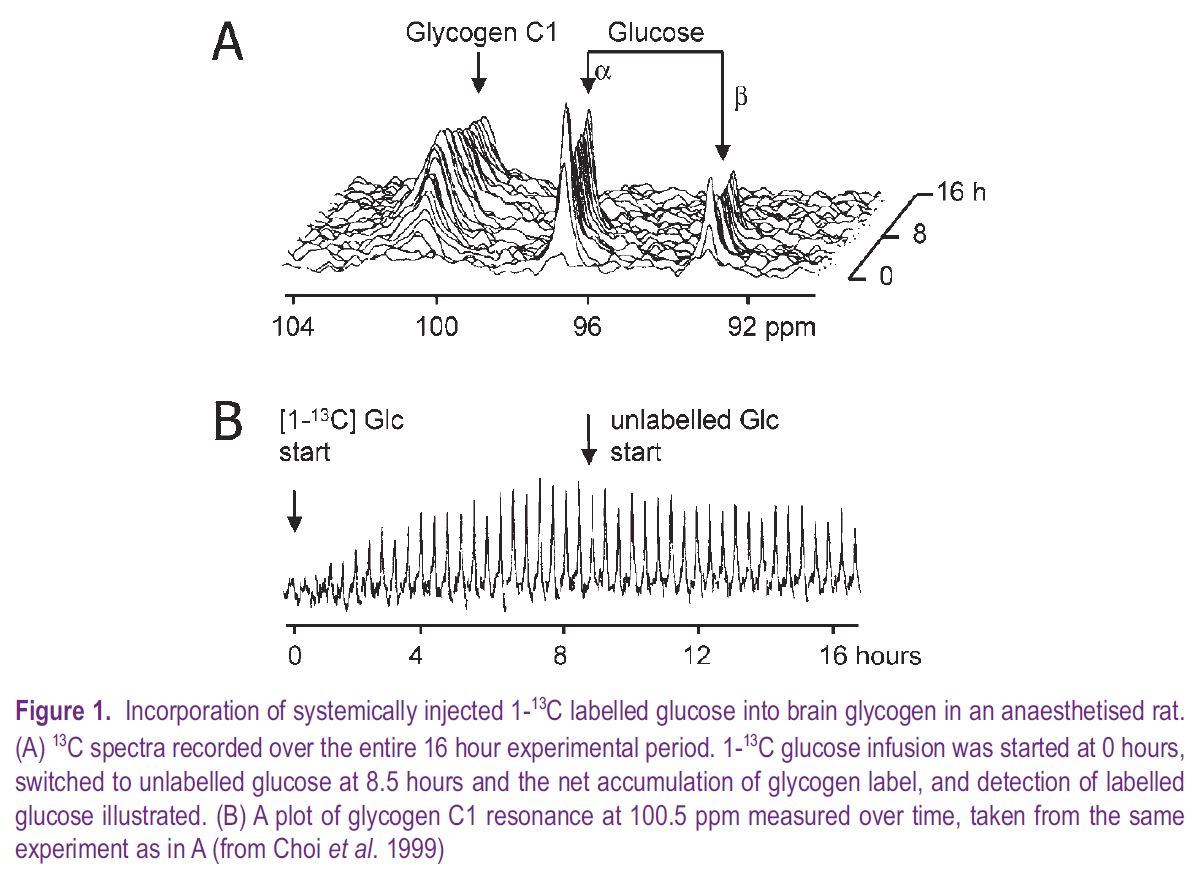
Glycogen and pathological conditions
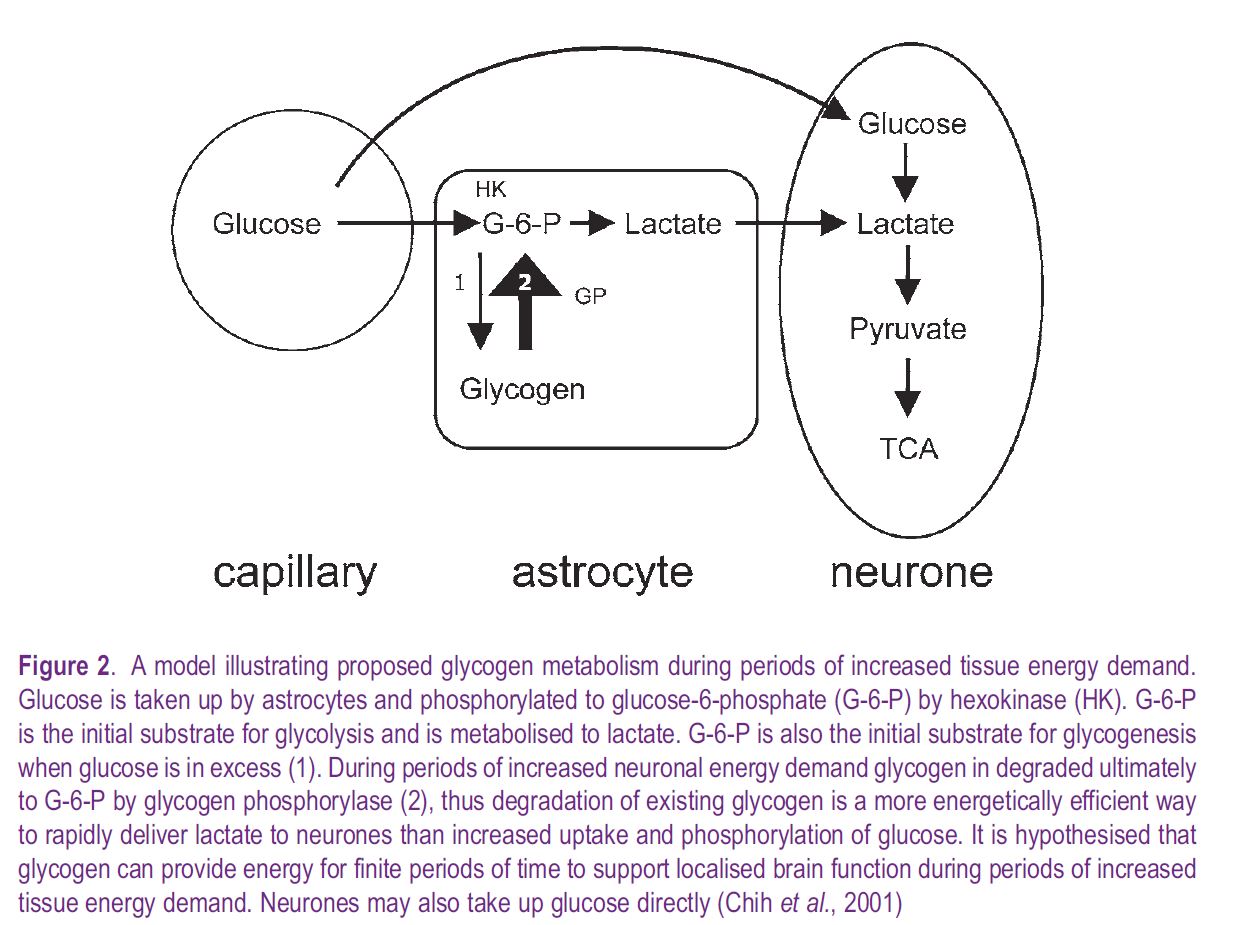
A consequence of the discovery of mammalian insulin in the early 1920s was the introduction of insulininduced systemic hypoglycaemia in the 1930s as a clinical therapy to alleviate symptoms of schizophrenia, a practice brutally depicted in the recent film A Beautiful Mind. Such clinical therapy prompted inquiries as to the effect of limiting glucose delivery on brain function and biochemistry. In adult dogs insulininduced hypoglycaemia led to decreases in brain glycogen initially in areas with the highest metabolic demand (Himwich, 1959), correlating brain glycogen metabolism with function. (In the mammalian brain there is clear evidence that astrocytic glycogen is metabolised to lactate, which is shuttled out of the astrocyte into the narrow extracellular space, then transported into neural elements where it is oxidatively metabolized, see Fig. 2). This ability of glycogen to support brain function for limited periods in the absence of glucose has since been demonstrated in the mouse optic nerve, where glycogen content at the onset of aglycaemia determined the duration of function during subsequent aglycaemia (Brown et al. 2003). NMR spectroscopic detection of 1-13C glucose incorporation into brain glycogen has been used to study the effects of hypoglycaemia in rat, for the first time allowing continual in vivo measurement of mammalian brain glycogen. These studies confirmed a hypoglycaemia-related decrease in brain glycogen, but only after brain glucose had fallen to zero (Choi et al. 2003). However, iatrogenic hypoglycaemia is a result of insulin therapy, and the rarity of spontaneous systemic hypoglycaemia suggests that brain glycogen’s primary role is not as an emergency energy reserve.
Glycogen and physiological conditions
A clue to the function of brain glycogen during physiological activity emerged from the Soviet Union over 50 years ago, with studies demonstrating that brain glycogen accumulated during sleep, decreased in a region-specific manner during sleep deprivation, and was mobilised upon waking, suggesting the awake brain degrades glycogen. The effects of general anaesthetics on brain glycogen provided supportive evidence. Under in vivo conditions sustained general anaesthesia resulted in elevated brain glycogen content, but general anaesthetics had no effect on glycogen levels of pure astrocyte cultures. Although a broad picture emerged of the conscious, awake brain constantly metabolising glycogen, what remained unclear was the specific conditions/stimuli that promoted brain glycogen degradation.
Sensory stimulation of rat face and vibrissae resulted in decreased glycogen in the sensorimotor cortex contralateral to the stimulus side, suggesting that sudden increases in focal energy demand are met by increased localised glycogenolysis (Swanson et al. 1992). Glycogen in the mouse optic nerve declined during intense physiological activity, and depletion of glycogen, or block of lactate transport, reduced function during periods of high intensity stimulus, even in the presence of normoglycaemic glucose. Glycogen therefore acts as an energy buffer, providing supplemental energy metabolite in the form of lactate during periods of increased tissue energy demand, when ambient normoglycaemic glucose is unable to meet immediate energy requirements (Brown et al. 2003). Thus, the following definition of a role for brain glycogen has emerged – to rapidly provide supplemental energy metabolite to support function during transient, localised increases in neuronal energy demand.
The future
Interest in brain glycogen has been re-awakened, encouraged by techniques that can measure in vivo brain glycogen for the first time, and evidence of a physiological role for brain glycogen. Key questions remaining unanswered include:
• How does the neurone signal to the astrocyte to initiate glycogen degradation?
• Does insulin regulate brain glycogen and, if so, is brain glycogen affected in type-1 diabetes?
• Is regional glycogen content correlated with specific brain activity?
References
Brown AM, Baltan Tekkök S & Ransom BR (2003). Glycogen regulation and functional role in mouse white matter. J Physiol 549, 501-512.
Chih C, Lipton P & Roberts EL (2001). Do active cerebral neurons really use lactate rather than glucose? Trends Neurosci 24, 573-578.
Choi IY, Seaquist ER & Gruetter R (2003). Effect of hypoglycemia on brain glycogen metabolism in vivo. J Neurosci Res 72, 25-32.
Choi IY, Tkac I, Ugurbil, K & Gruetter R (1999). Noninvasive measurements of [1-13C]glycogen concentrations and metabolism in rat brain in vivo. J Neurochem 73, 1300-1308.
Himwich WA (1959). Biochemical changes in the brain occurring during insulin hypoglycaemia. In Insulin Treatment in Psychiatry. Ed. Rinkel M & Himwich, HE, pp. 85-105. Philosophical Library, New York
Swanson RA, Morton MM, Sagar SM & Sharp FR (1992). Sensory stimulation induces local cerebral glycogenolysis: demonstration by autoradiography. Neuroscience 51, 451-461.
Vannucci SJ & Simpson IA (2001). Synopsis of the workshop entitled “Is lactate a nutrient for neurons” held at the Brain Energy Meeting in Trondheim, Norway. J Neurosci Res 66, 821-823.
